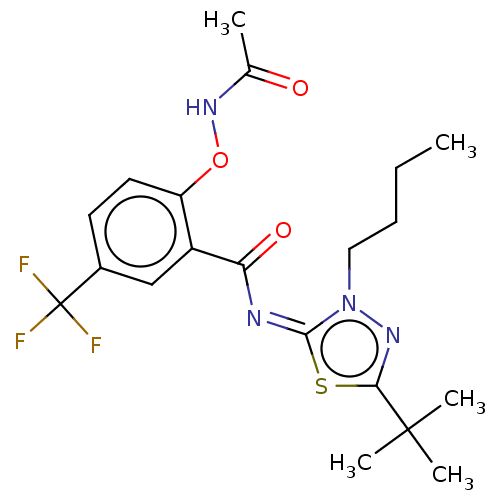
Affinity DataKi: 398nM ΔG°: -36.2kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nM ΔG°: -33.9kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 1.26E+3nM ΔG°: -33.3kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nM ΔG°: -32.2kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 5.01E+3nM ΔG°: -29.9kJ/molepH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DatapH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DatapH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DatapH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DatapH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DatapH: 7.2 T: 2°CAssay Description:IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand displacement experiments. The inhibit...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nM ΔG°: -55.3kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nM ΔG°: -55.3kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMpH: 7.4Assay Description:HEK293 cells stably expressing rat CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogenized...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nM ΔG°: -55.3kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nM ΔG°: -55.3kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.320nM ΔG°: -55.1kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.400nM ΔG°: -54.5kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.400nM ΔG°: -54.5kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.400nM ΔG°: -54.5kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.450nM ΔG°: -54.2kJ/molepH: 7.4 T: 2°CAssay Description:In this assay, 18 ug of crude membrane expressing hCB1 or hCB2 receptors were re-suspended in 300 uL of binding buffer containing 50 mM Tris-HCl, 2.5...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nM ΔG°: -54.0kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nM ΔG°: -54.0kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nM ΔG°: -54.0kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nM ΔG°: -54.0kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nM ΔG°: -54.0kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nM ΔG°: -54.0kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nM ΔG°: -54.0kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMpH: 7.4Assay Description:HEK293 cells stably expressing rat CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogenized...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMpH: 7.4Assay Description:HEK293 cells stably expressing rat CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogenized...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nM ΔG°: -53.5kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nM ΔG°: -53.5kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nM ΔG°: -53.5kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMpH: 7.4Assay Description:HEK293 cells stably expressing rat CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogenized...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nM ΔG°: -53.1kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nM ΔG°: -53.1kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nMpH: 7.4Assay Description:HEK293 cells stably expressing rat CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogenized...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nM ΔG°: -53.1kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nMpH: 7.4Assay Description:HEK293 cells stably expressing rat CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogenized...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nM ΔG°: -53.1kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nMpH: 7.4Assay Description:HEK293 cells stably expressing rat CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogenized...More data for this Ligand-Target Pair
Affinity DataKi: 0.800nM ΔG°: -52.8kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.800nM ΔG°: -52.8kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.800nMpH: 7.4Assay Description:HEK293 cells stably expressing rat CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogenized...More data for this Ligand-Target Pair
Affinity DataKi: 0.800nMpH: 7.4Assay Description:HEK293 cells stably expressing rat CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogenized...More data for this Ligand-Target Pair
Affinity DataKi: 0.900nMpH: 7.4Assay Description:HEK293 cells stably expressing rat CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogenized...More data for this Ligand-Target Pair
Affinity DataKi: 0.900nMpH: 7.4Assay Description:HEK293 cells stably expressing rat CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogenized...More data for this Ligand-Target Pair
Affinity DataKi: 0.900nM ΔG°: -52.5kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.900nM ΔG°: -52.5kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 0.900nM ΔG°: -52.5kJ/molepH: 7.4 T: 2°CAssay Description:HEK293 cells stably expressing human CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogeniz...More data for this Ligand-Target Pair
Affinity DataKi: 1nMpH: 7.4Assay Description:HEK293 cells stably expressing rat CB2 receptors were grown until a confluent monolayer was formed. Briefly, the cells were harvested and homogenized...More data for this Ligand-Target Pair

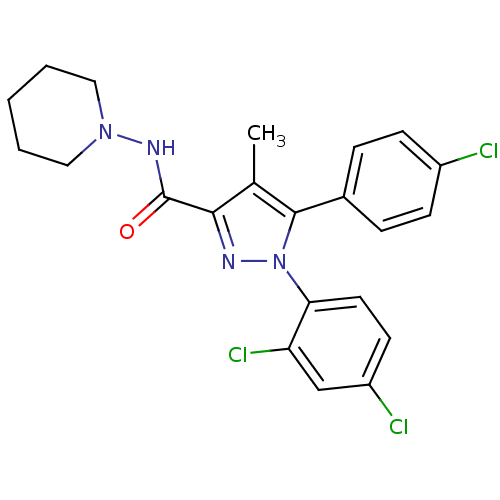
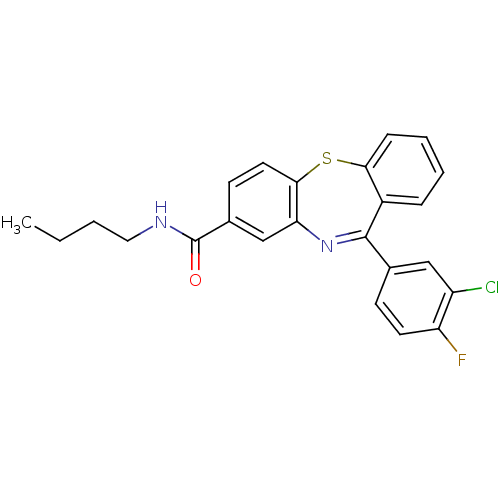
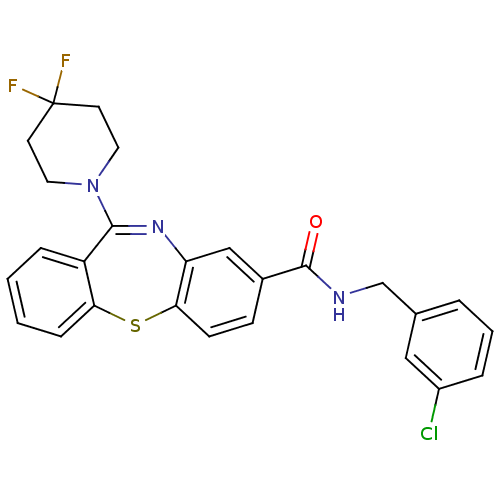
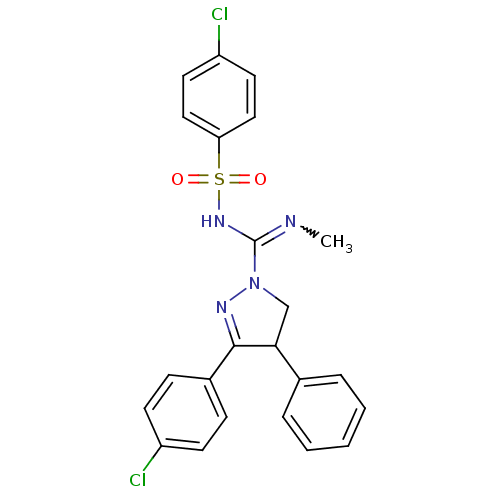

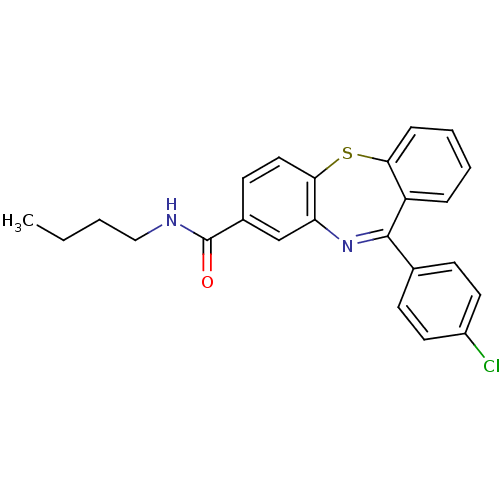
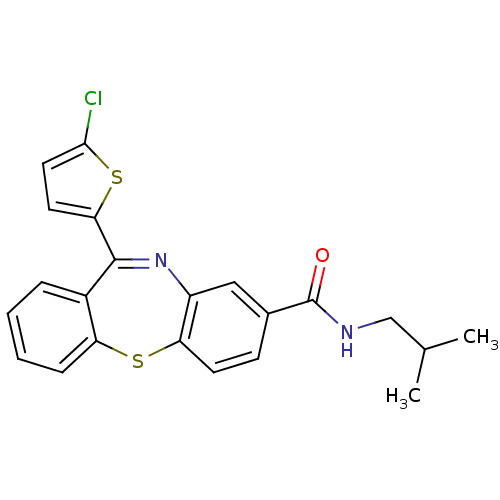
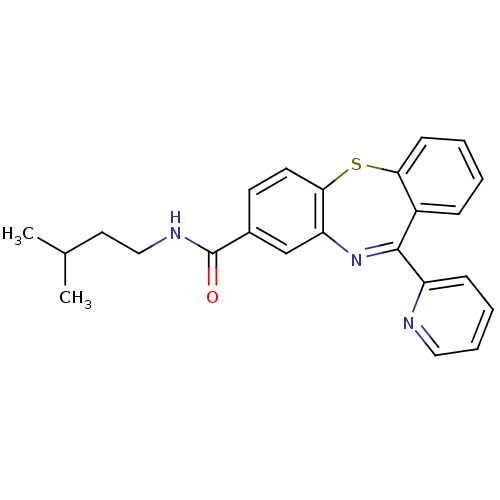

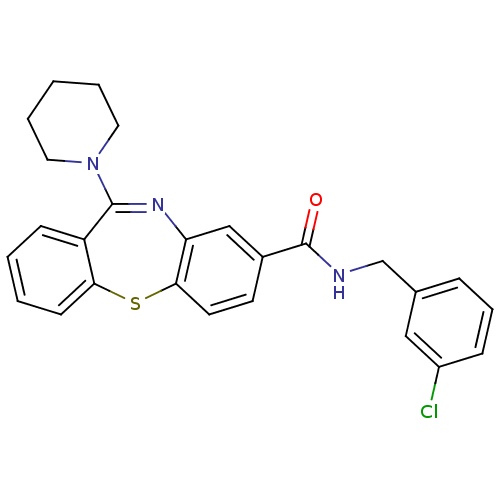





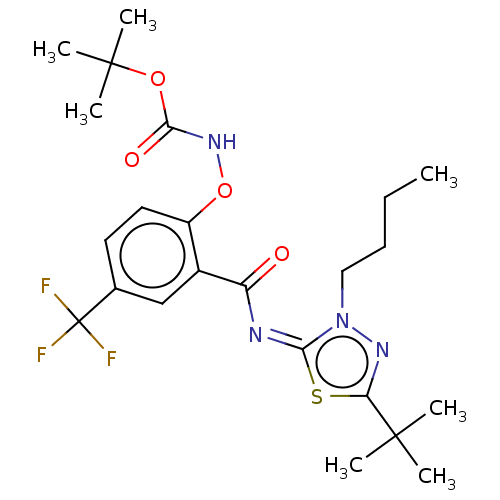


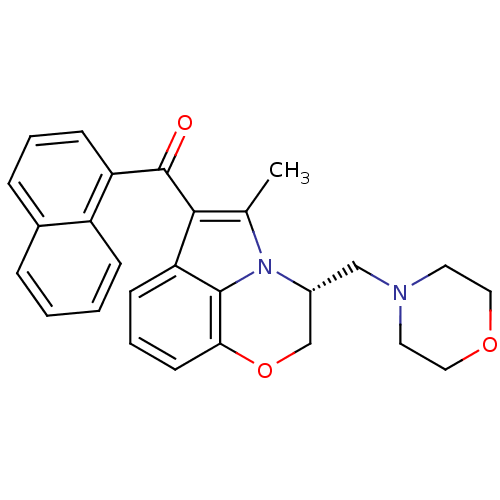
 3D Structure (crystal)
3D Structure (crystal)