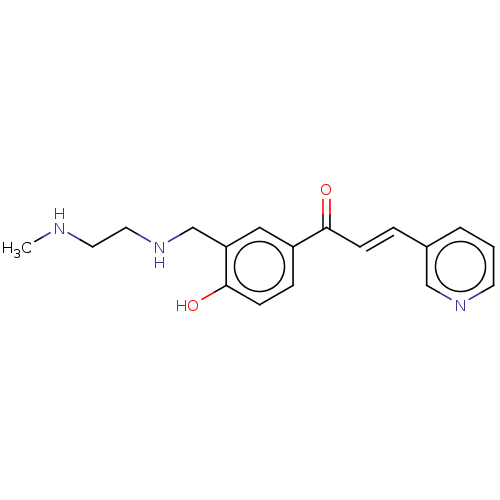
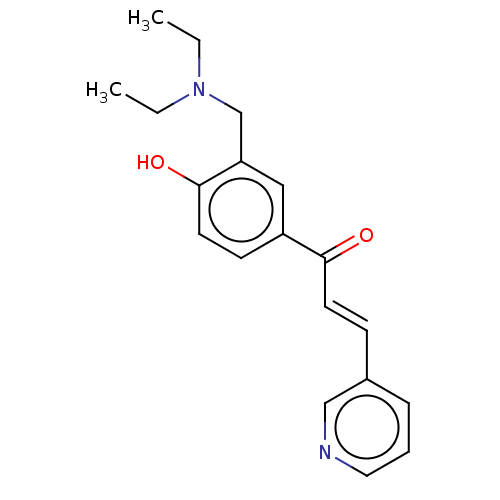
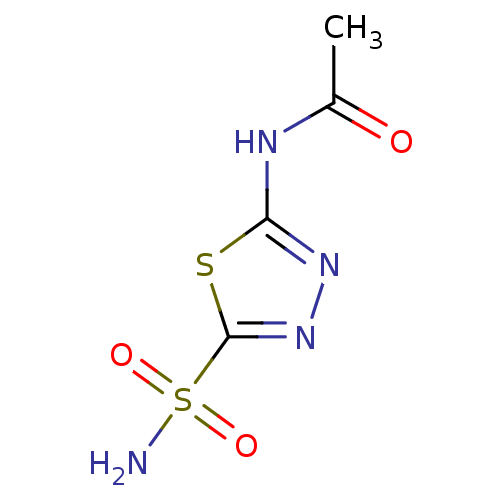
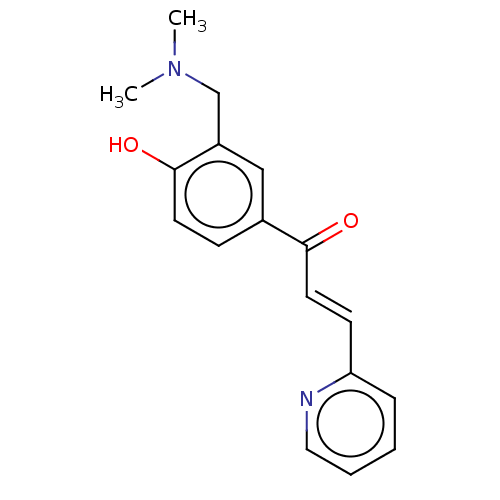
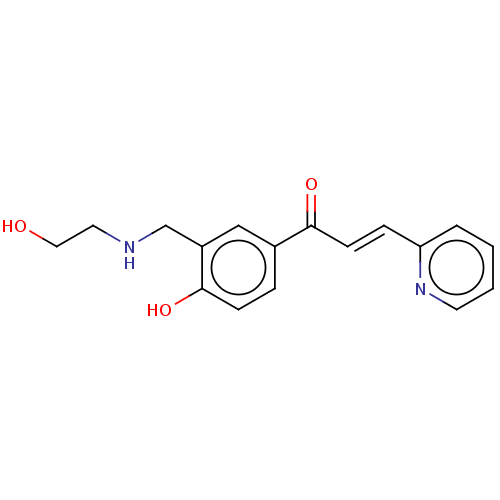
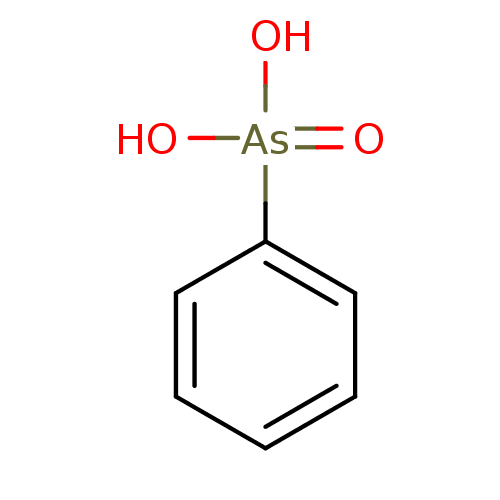
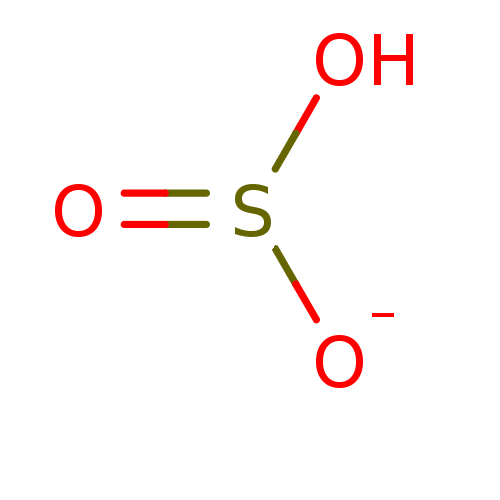
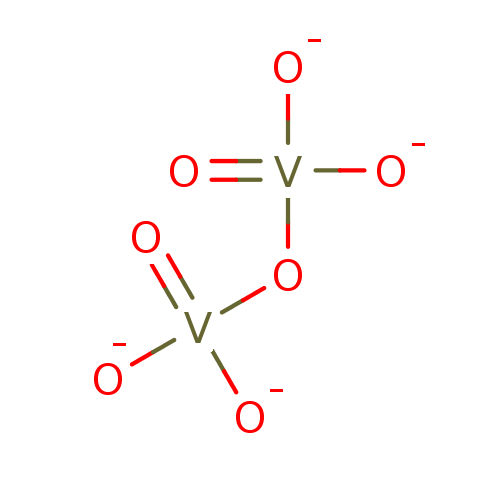
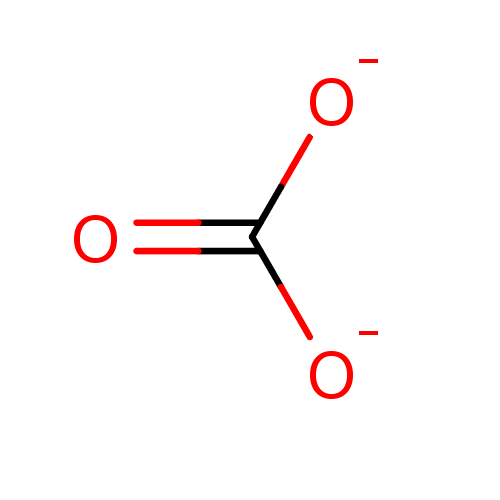
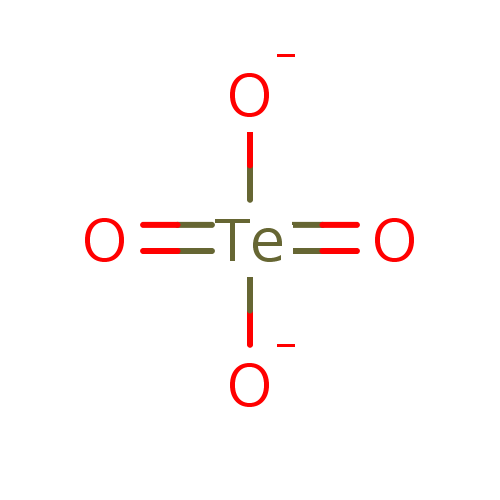
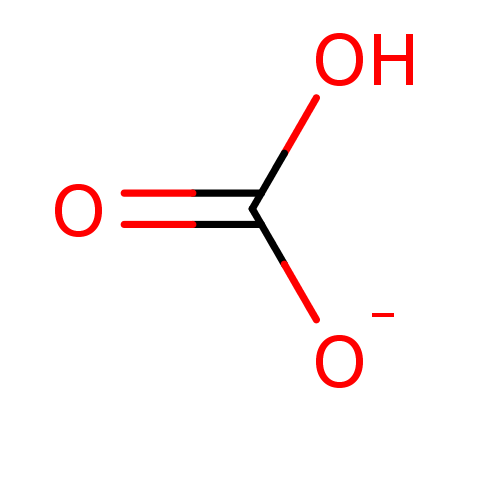
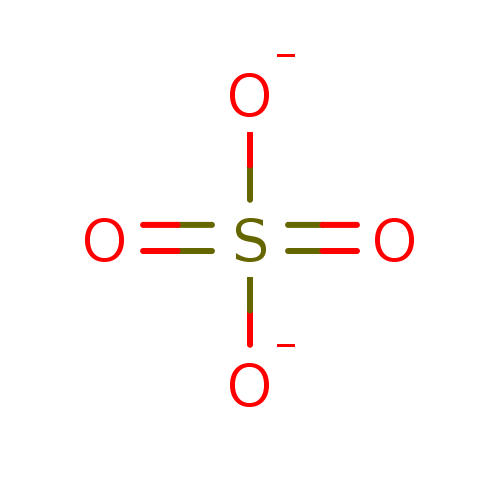
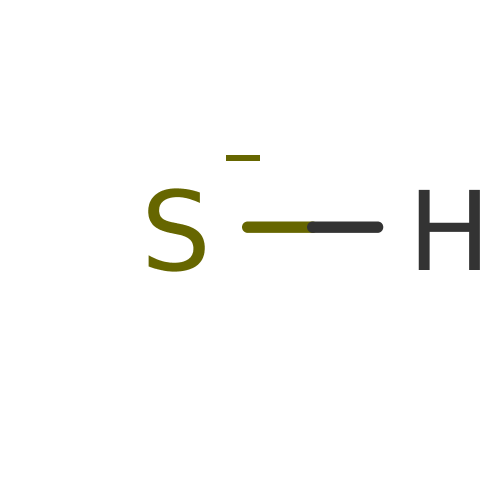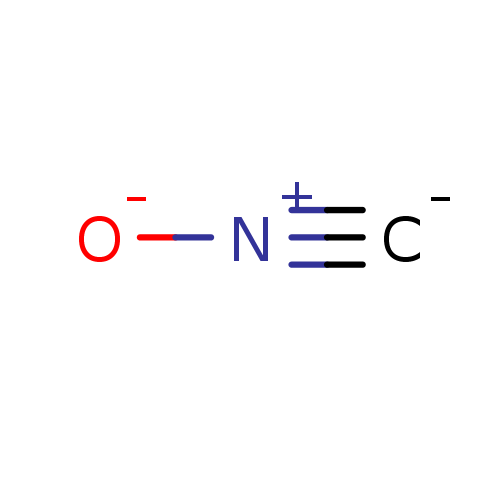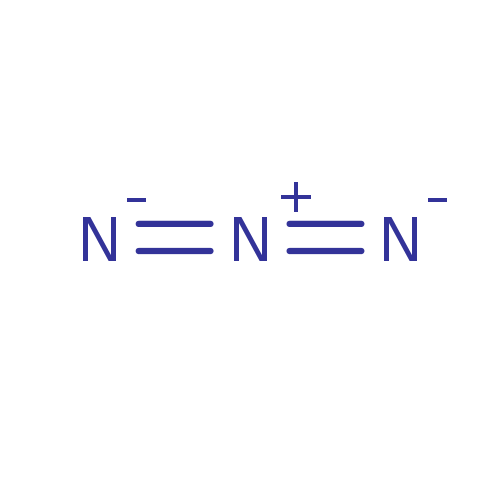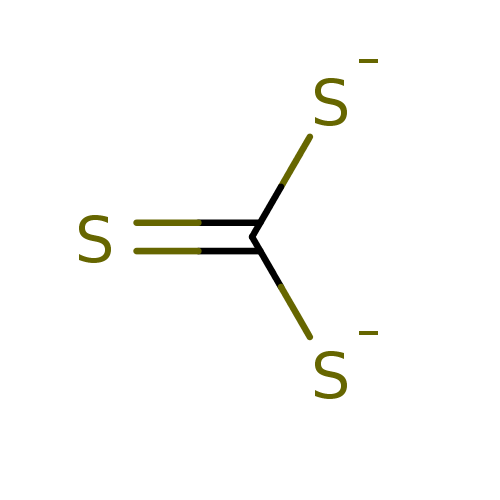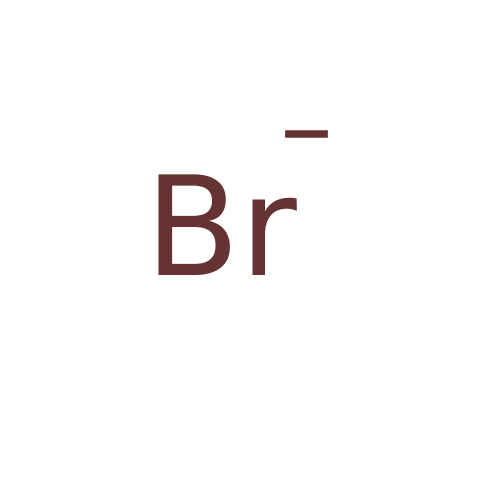Affinity DataKi: 4.90E+3nM ΔG°: -30.3kJ/molepH: 7.4 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity [Khalifah et al., J. Biol. Chem., ...More data for this Ligand-Target Pair
Affinity DataKi: 2.35E+4nM ΔG°: -26.4kJ/molepH: 7.4 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity [Khalifah et al., J. Biol. Chem., ...More data for this Ligand-Target Pair
Affinity DataKi: 6.50E+4nM ΔG°: -23.9kJ/molepH: 7.4 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity [Khalifah et al., J. Biol. Chem., ...More data for this Ligand-Target Pair
Affinity DataKi: 6.58E+4nM ΔG°: -23.9kJ/molepH: 7.4 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity [Khalifah et al., J. Biol. Chem., ...More data for this Ligand-Target Pair
Affinity DataKi: 7.65E+4nM ΔG°: -23.5kJ/molepH: 7.4 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity [Khalifah et al., J. Biol. Chem., ...More data for this Ligand-Target Pair
Affinity DataKi: 7.65E+4nM ΔG°: -23.5kJ/molepH: 7.4 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity [Khalifah et al., J. Biol. Chem., ...More data for this Ligand-Target Pair
Affinity DataKi: 7.71E+4nM ΔG°: -23.5kJ/molepH: 7.4 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity [Khalifah et al., J. Biol. Chem., ...More data for this Ligand-Target Pair
Affinity DataKi: 7.91E+4nM ΔG°: -23.4kJ/molepH: 7.4 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity [Khalifah et al., J. Biol. Chem., ...More data for this Ligand-Target Pair
Affinity DataKi: 7.95E+4nM ΔG°: -23.4kJ/molepH: 7.4 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity [Khalifah et al., J. Biol. Chem., ...More data for this Ligand-Target Pair
Affinity DataKi: 8.19E+4nM ΔG°: -23.3kJ/molepH: 7.4 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity [Khalifah et al., J. Biol. Chem., ...More data for this Ligand-Target Pair
Affinity DataKi: 8.26E+4nM ΔG°: -23.3kJ/molepH: 7.4 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity [Khalifah et al., J. Biol. Chem., ...More data for this Ligand-Target Pair
Affinity DataKi: 8.58E+4nM ΔG°: -23.2kJ/molepH: 7.4 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity [Khalifah et al., J. Biol. Chem., ...More data for this Ligand-Target Pair
Affinity DataKi: 9.01E+4nM ΔG°: -23.1kJ/molepH: 7.4 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity [Khalifah et al., J. Biol. Chem., ...More data for this Ligand-Target Pair
Affinity DataKi: 9.54E+4nM ΔG°: -22.9kJ/molepH: 7.4 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity [Khalifah et al., J. Biol. Chem., ...More data for this Ligand-Target Pair
Affinity DataKi: 6.80nM ΔG°: -45.8kJ/molepH: 7.5 T: 2°CAssay Description:Inhibition of Flaveria bidentis carbonic anhydrase 1 preincubated for 15 mins by stopped-flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 21nM ΔG°: -43.1kJ/molepH: 7.5 T: 2°CAssay Description:Inhibition of Vibrio cholerae alpha carbonic anhydrase preincubated for 15 mins by stopped-flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 63nM ΔG°: -40.4kJ/molepH: 7.5 T: 2°CAssay Description:Inhibition of Methanosarcina thermophila gamma carbonic anhydrase preincubated for 15 mins by stopped-flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 104nMpH: 7.5Assay Description:Inhibition of recombinant Mycobacterium tuberculosis Rv3273 carbonic anhydrase after 15 mins by stopped flow CO2 hydration assay at pH 7.5More data for this Ligand-Target Pair
Affinity DataKi: 473nM ΔG°: -35.5kJ/molepH: 7.5 T: 2°CAssay Description:Inhibition of Vibrio cholerae recombinant carbonic anhydrase gamma expressed in Escherichia coli DE3 cells preincubated for 15 mins by stopped-flow C...More data for this Ligand-Target Pair
Affinity DataKi: 7.00E+3nM ΔG°: -28.9kJ/molepH: 7.5 T: 2°CAssay Description:Inhibition of Flaveria bidentis carbonic anhydrase 1 preincubated for 15 mins by stopped-flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 7.40E+3nM ΔG°: -29.3kJ/molepH: 7.5 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity Khalifah RG. The carbon dioxide hy...More data for this Ligand-Target Pair
Affinity DataKi: 8.00E+3nM ΔG°: -28.6kJ/molepH: 7.5 T: 2°CAssay Description:Inhibition of Flaveria bidentis carbonic anhydrase 1 preincubated for 15 mins by stopped-flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.30E+4nM ΔG°: -26.0kJ/molepH: 7.5 T: 2°CAssay Description:Inhibition of Flaveria bidentis carbonic anhydrase 1 preincubated for 15 mins by stopped-flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.10E+4nM ΔG°: -25.3kJ/molepH: 7.5 T: 2°CAssay Description:Inhibition of Flaveria bidentis carbonic anhydrase 1 preincubated for 15 mins by stopped-flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.30E+4nM ΔG°: -25.1kJ/molepH: 7.5 T: 2°CAssay Description:Inhibition of Flaveria bidentis carbonic anhydrase 1 preincubated for 15 mins by stopped-flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 4.30E+4nM ΔG°: -24.5kJ/molepH: 7.5 T: 2°CAssay Description:Inhibition of Flaveria bidentis carbonic anhydrase 1 preincubated for 15 mins by stopped-flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 5.40E+4nM ΔG°: -23.9kJ/molepH: 7.5 T: 2°CAssay Description:Inhibition of Vibrio cholerae carbonic anhydrase beta preincubated for 15 mins by stopped-flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 6.80E+4nM ΔG°: -23.4kJ/molepH: 7.5 T: 2°CAssay Description:Inhibition of Flaveria bidentis carbonic anhydrase 1 preincubated for 15 mins by stopped-flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 7.20E+4nM ΔG°: -23.6kJ/molepH: 7.5 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity Khalifah RG. The carbon dioxide hy...More data for this Ligand-Target Pair
Affinity DataKi: 7.30E+4nM ΔG°: -23.6kJ/molepH: 7.5 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity Khalifah RG. The carbon dioxide hy...More data for this Ligand-Target Pair
Affinity DataKi: 7.40E+4nM ΔG°: -23.2kJ/molepH: 7.5 T: 2°CAssay Description:Inhibition of Flaveria bidentis carbonic anhydrase 1 preincubated for 15 mins by stopped-flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 7.50E+4nM ΔG°: -23.1kJ/molepH: 7.5 T: 2°CAssay Description:Inhibition of Flaveria bidentis carbonic anhydrase 1 preincubated for 15 mins by stopped-flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 7.60E+4nM ΔG°: -23.1kJ/molepH: 7.5 T: 2°CAssay Description:Inhibition of Flaveria bidentis carbonic anhydrase 1 preincubated for 15 mins by stopped-flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 7.90E+4nM ΔG°: -23.0kJ/molepH: 7.5 T: 2°CAssay Description:Inhibition of Vibrio cholerae carbonic anhydrase beta preincubated for 15 mins by stopped-flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 8.50E+4nM ΔG°: -22.8kJ/molepH: 7.5 T: 2°CAssay Description:Inhibition of Vibrio cholerae carbonic anhydrase beta preincubated for 15 mins by stopped-flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 8.60E+4nM ΔG°: -22.8kJ/molepH: 7.5 T: 2°CAssay Description:Inhibition of Vibrio cholerae carbonic anhydrase beta preincubated for 15 mins by stopped-flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 8.80E+4nM ΔG°: -22.8kJ/molepH: 7.5 T: 2°CAssay Description:Inhibition of Flaveria bidentis carbonic anhydrase 1 preincubated for 15 mins by stopped-flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 9.20E+4nM ΔG°: -23.0kJ/molepH: 7.5 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity Khalifah RG. The carbon dioxide hy...More data for this Ligand-Target Pair
Affinity DataKi: 9.40E+4nM ΔG°: -23.0kJ/molepH: 7.5 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity Khalifah RG. The carbon dioxide hy...More data for this Ligand-Target Pair
Affinity DataKi: 1.80E+5nM ΔG°: -21.4kJ/molepH: 7.5 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity Khalifah RG. The carbon dioxide hy...More data for this Ligand-Target Pair
Affinity DataKi: 2.10E+5nM ΔG°: -21.0kJ/molepH: 7.5 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity Khalifah RG. The carbon dioxide hy...More data for this Ligand-Target Pair
Affinity DataKi: 3.70E+5nM ΔG°: -19.6kJ/molepH: 7.5 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity Khalifah RG. The carbon dioxide hy...More data for this Ligand-Target Pair
Affinity DataKi: 3.80E+5nM ΔG°: -19.5kJ/molepH: 7.5 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity Khalifah RG. The carbon dioxide hy...More data for this Ligand-Target Pair
Affinity DataKi: 4.20E+5nM ΔG°: -19.3kJ/molepH: 7.5 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity Khalifah RG. The carbon dioxide hy...More data for this Ligand-Target Pair
Affinity DataKi: 4.50E+5nM ΔG°: -19.1kJ/molepH: 7.5 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity Khalifah RG. The carbon dioxide hy...More data for this Ligand-Target Pair
Affinity DataKi: 4.80E+5nM ΔG°: -18.9kJ/molepH: 7.5 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity Khalifah RG. The carbon dioxide hy...More data for this Ligand-Target Pair
Affinity DataKi: 5.00E+5nM ΔG°: -18.8kJ/molepH: 7.5 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity Khalifah RG. The carbon dioxide hy...More data for this Ligand-Target Pair
Affinity DataKi: 5.40E+5nM ΔG°: -18.7kJ/molepH: 7.5 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity Khalifah RG. The carbon dioxide hy...More data for this Ligand-Target Pair
Affinity DataKi: 5.60E+5nM ΔG°: -18.6kJ/molepH: 7.5 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity Khalifah RG. The carbon dioxide hy...More data for this Ligand-Target Pair
Affinity DataKi: 5.70E+5nM ΔG°: -18.5kJ/molepH: 7.5 T: 2°CAssay Description:An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity Khalifah RG. The carbon dioxide hy...More data for this Ligand-Target Pair