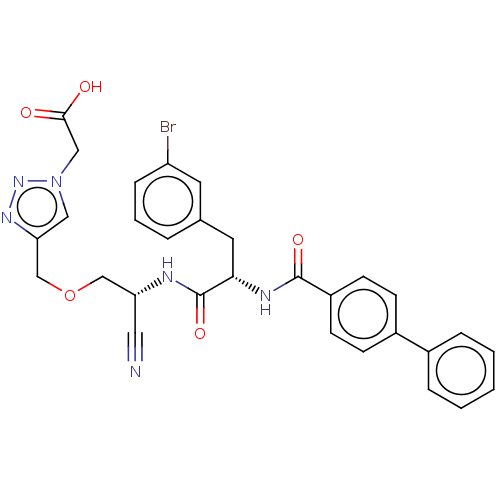
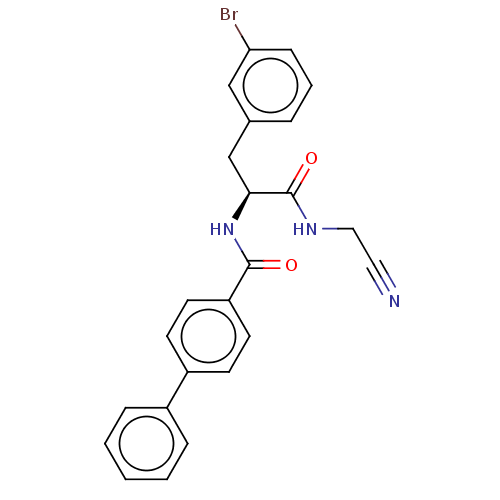
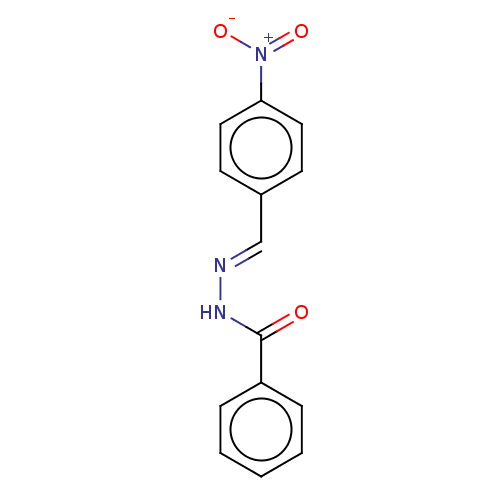
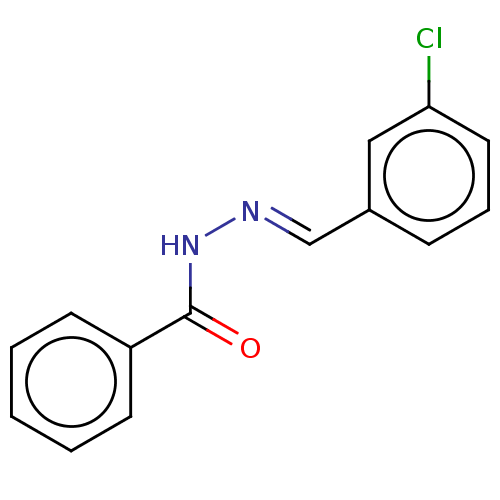
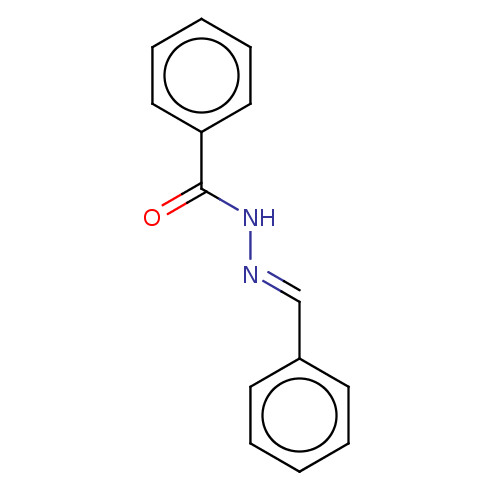
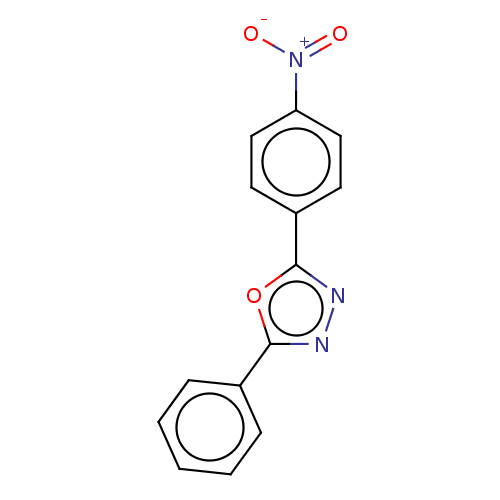
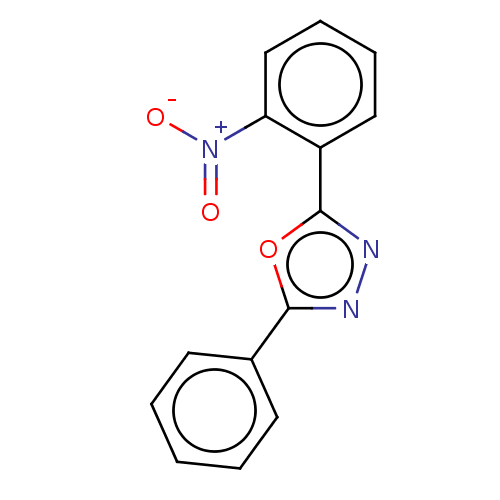
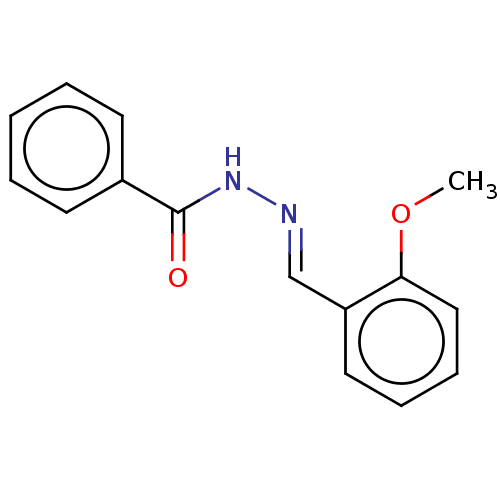
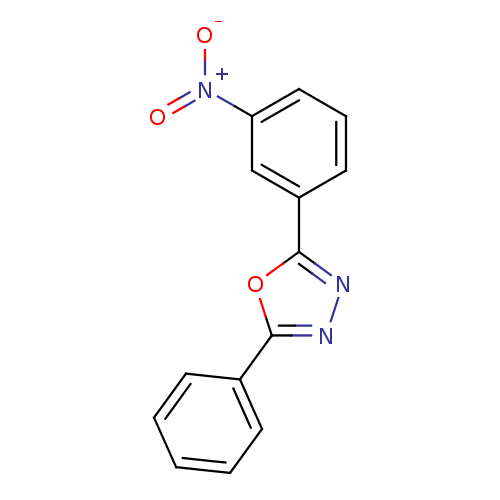
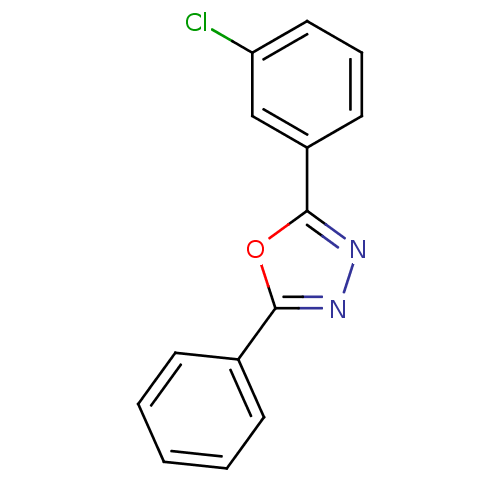
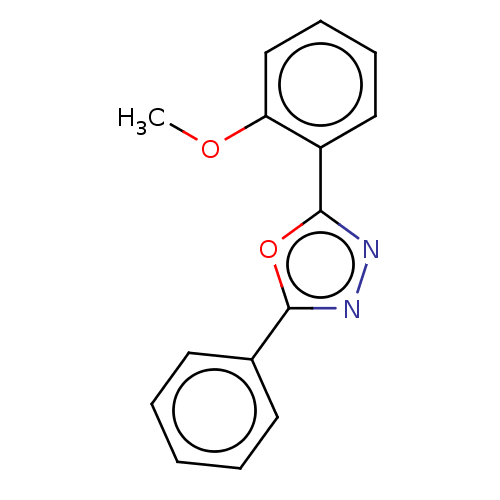
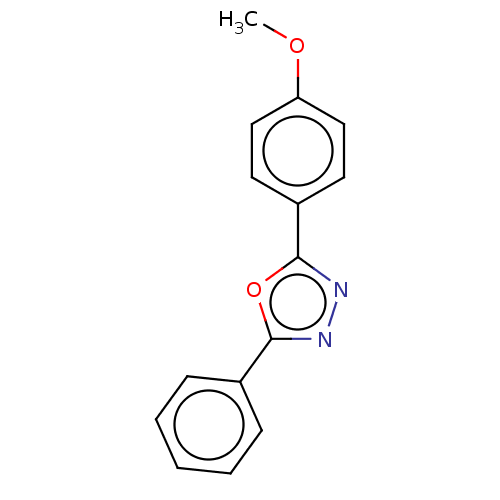
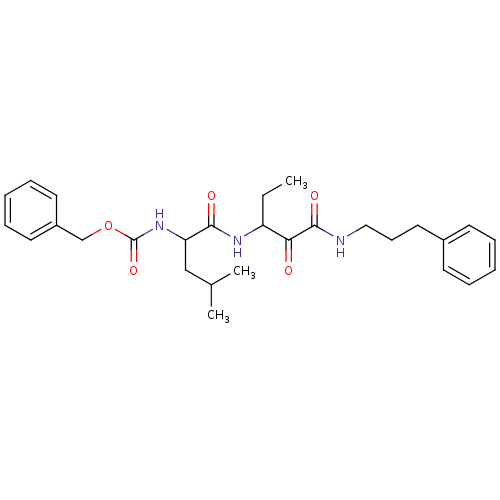
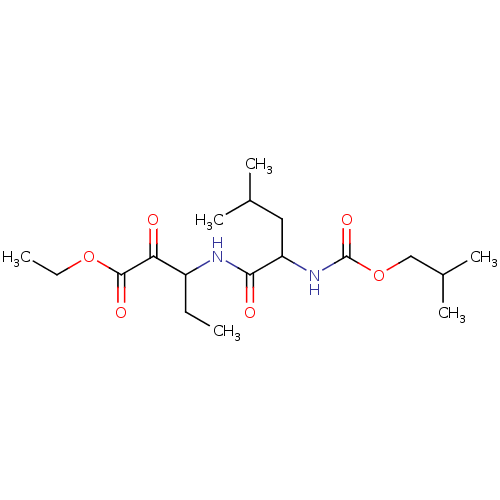
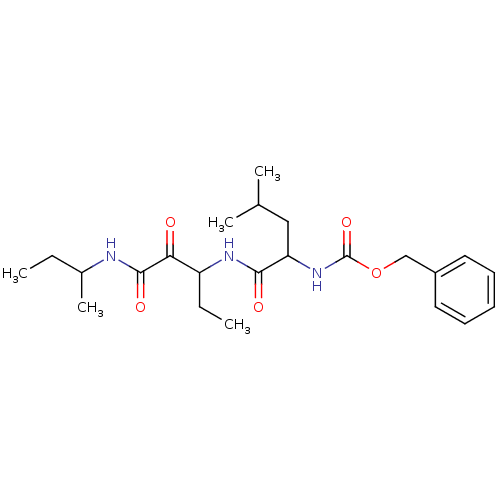
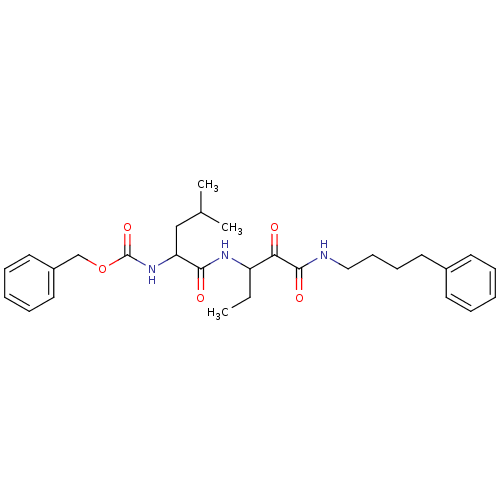
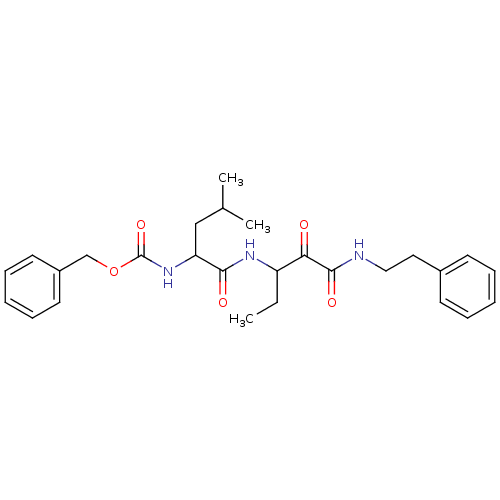
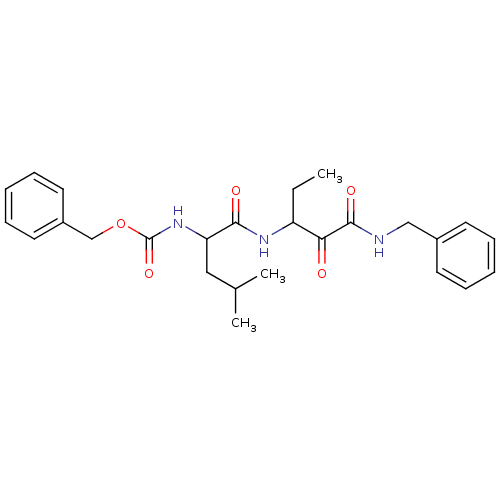
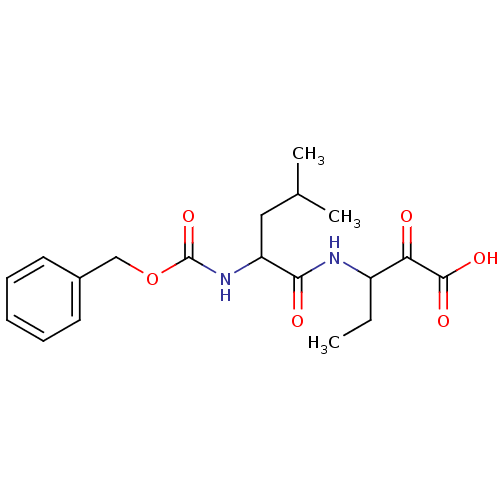
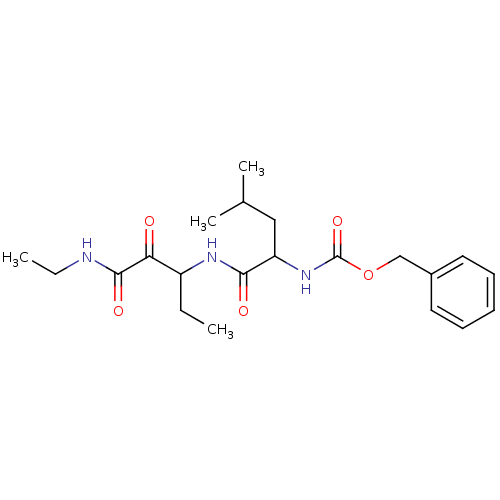
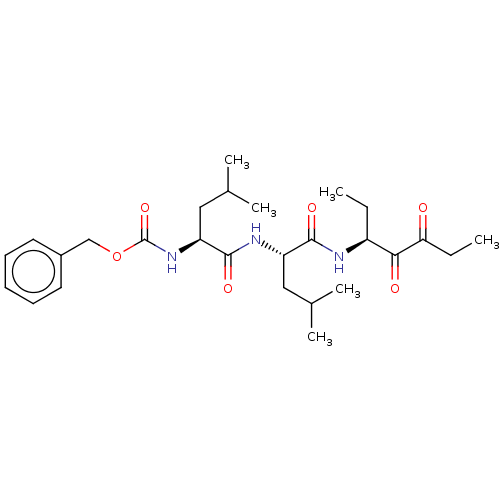
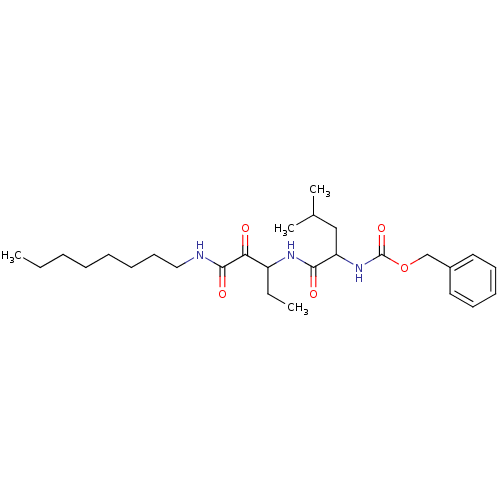
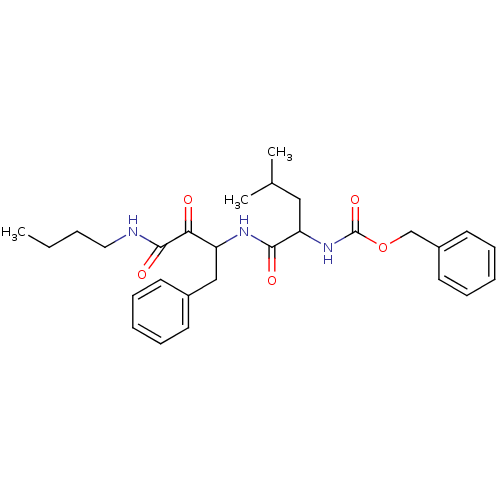
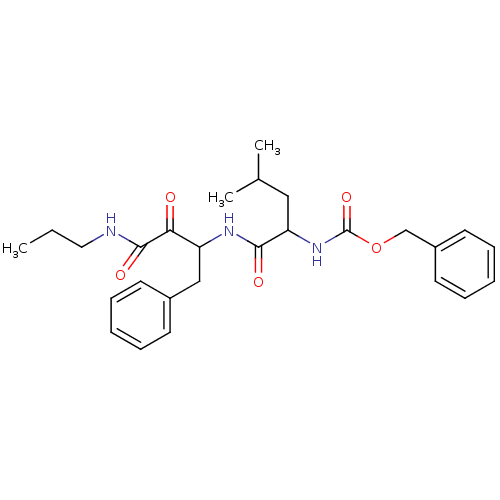
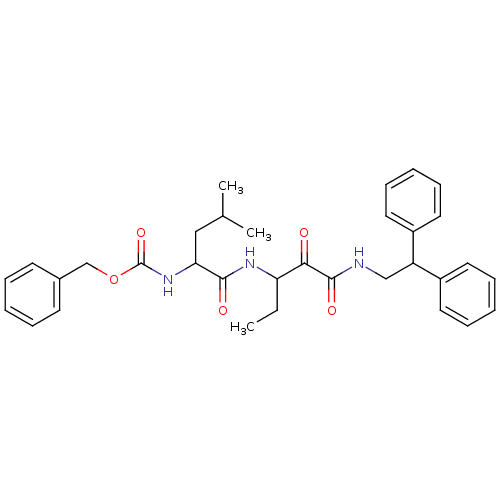
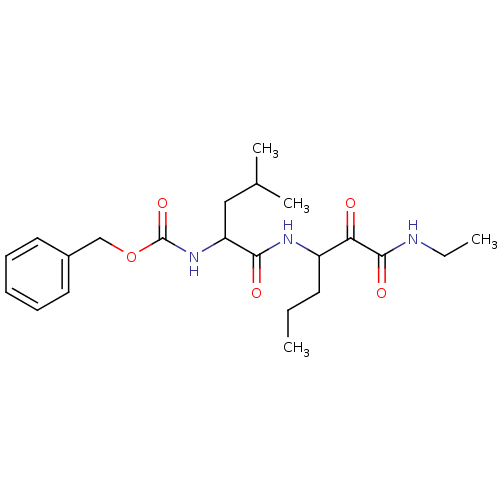
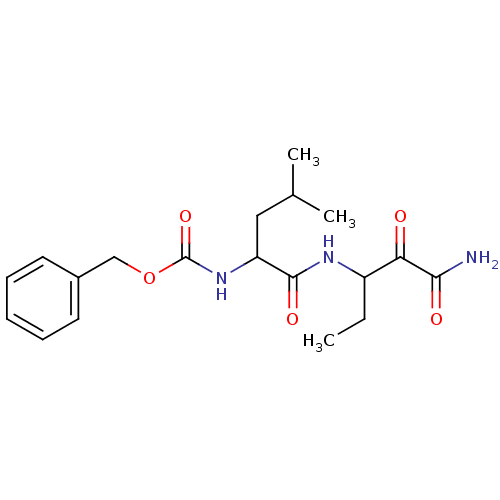
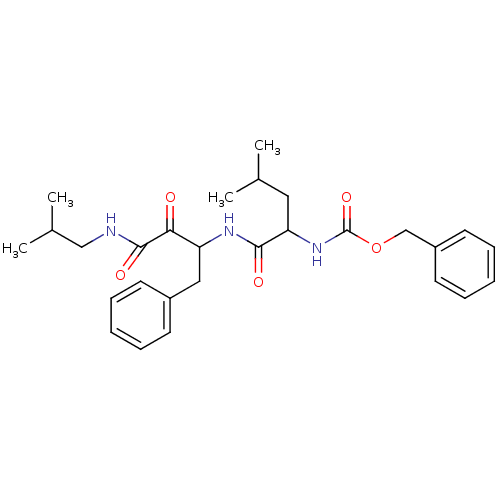
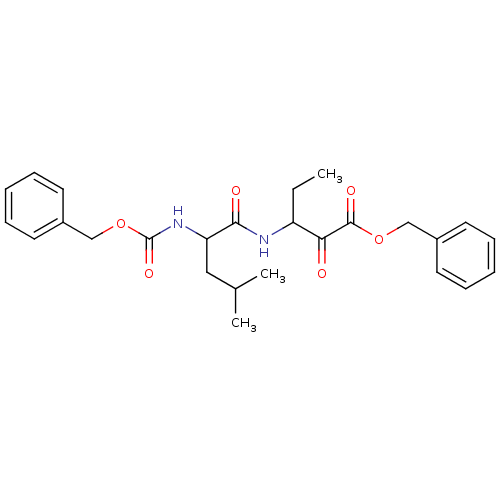
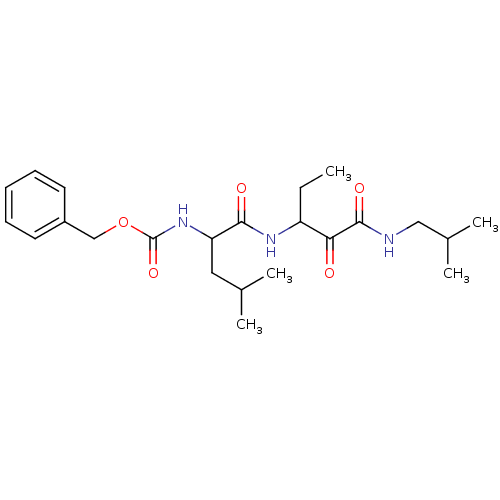
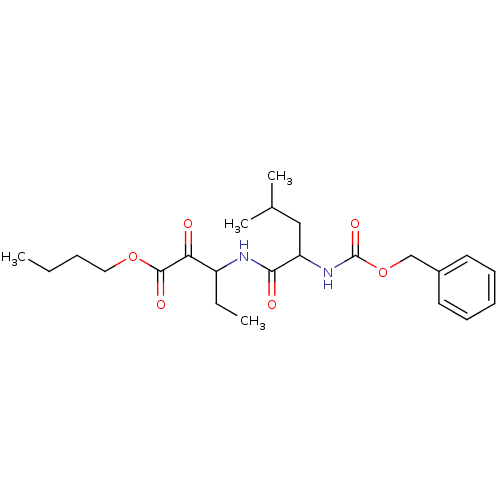
Affinity DataKi: 19nMpH: 4.5Assay Description:Inhibition of human liver cathepsin B using Abz-Gly-Ile-Val-Arg-Ala-Lys(Dnp)-OH as substrate at pH 4.5 incubated for 30 mins measured for 10 mins by ...More data for this Ligand-Target Pair
Affinity DataKi: 27nMpH: 4.5Assay Description:Inhibition of human liver cathepsin B using Cbz-Arg-Arg-pNA as substrate at pH 4.5 incubated for 30 mins measured for 20 mins by photometrical analys...More data for this Ligand-Target Pair
Affinity DataKi: 131nMpH: 4.5Assay Description:Inhibition of human liver cathepsin B using Abz-Gly-Ile-Val-Arg-Ala-Lys(Dnp)-OH as substrate at pH 4.5 incubated for 30 mins measured for 10 mins by ...More data for this Ligand-Target Pair
Affinity DataKi: 132nMpH: 4.5Assay Description:Inhibition of human liver cathepsin B using Abz-Gly-Ile-Val-Arg-Ala-Lys(Dnp)-OH as substrate at pH 4.5 incubated for 30 mins measured for 10 mins by ...More data for this Ligand-Target Pair
Affinity DataKi: 136nMpH: 4.5Assay Description:Inhibition of human liver cathepsin B using Cbz-Arg-Arg-pNA as substrate at pH 4.5 incubated for 30 mins measured for 20 mins by photometrical analys...More data for this Ligand-Target Pair
Affinity DataKi: 205nMpH: 4.5Assay Description:Inhibition of human liver cathepsin B using Cbz-Arg-Arg-pNA as substrate at pH 4.5 incubated for 30 mins measured for 20 mins by photometrical analys...More data for this Ligand-Target Pair
Affinity DataKi: 368nMpH: 4.5Assay Description:Inhibition of human liver cathepsin B using Abz-Gly-Ile-Val-Arg-Ala-Lys(Dnp)-OH as substrate at pH 4.5 incubated for 30 mins measured for 10 mins by ...More data for this Ligand-Target Pair
Affinity DataKi: 420nMpH: 4.5Assay Description:Inhibition of human liver cathepsin B using Cbz-Arg-Arg-pNA as substrate at pH 4.5 incubated for 30 mins measured for 20 mins by photometrical analys...More data for this Ligand-Target Pair
Affinity DataKi: 707nMpH: 4.5Assay Description:Inhibition of human liver cathepsin B using Cbz-Arg-Arg-pNA as substrate at pH 4.5 incubated for 30 mins measured for 20 mins by photometrical analys...More data for this Ligand-Target Pair
Affinity DataKi: 113nM ΔG°: -41.2kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 120nM ΔG°: -41.1kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 140nM ΔG°: -40.7kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 180nM ΔG°: -40.0kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 190nM ΔG°: -39.9kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 210nM ΔG°: -39.6kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 260nM ΔG°: -39.1kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 290nM ΔG°: -38.8kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 440nM ΔG°: -37.7kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 664nM ΔG°: -36.7kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 790nM ΔG°: -36.2kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 830nM ΔG°: -36.1kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nM ΔG°: -35.6kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 1.28E+3nM ΔG°: -35.0kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 1.62E+3nM ΔG°: -34.4kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 1.92E+3nM ΔG°: -33.9kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 2.69E+3nM ΔG°: -33.1kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 4.44E+3nM ΔG°: -31.8kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 6.85E+3nM ΔG°: -30.7kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 8.72E+3nM ΔG°: -30.0kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 9.22E+3nM ΔG°: -29.9kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 1.13E+4nM ΔG°: -29.4kJ/molepH: 5.0 T: 2°CAssay Description:The proteolytic activity was estimated at pH 5.0, 37 °C using 0.1 M acetate buffer as the incubation medium. The homogenate prepared above was incuba...More data for this Ligand-Target Pair
Affinity DataKi: 200nMpH: 5.2Assay Description:Inhibition of alpha-keto esters towards cathepsin B at pH 5.2More data for this Ligand-Target Pair
Affinity DataKi: 400nMpH: 5.2Assay Description:Inhibition of alpha-keto esters towards cathepsin B at pH 5.2More data for this Ligand-Target Pair
Affinity DataKi: 500nMpH: 5.2Assay Description:Inhibition of alpha-keto esters towards cathepsin B at pH 5.2More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nMpH: 5.2Assay Description:Inhibition of alpha-keto esters towards cathepsin B at pH 5.2More data for this Ligand-Target Pair
Affinity DataKi: 1.30E+3nMpH: 5.2Assay Description:Inhibition of alpha-keto esters towards cathepsin B at pH 5.2More data for this Ligand-Target Pair
Affinity DataKi: 1.30E+3nMpH: 5.2Assay Description:Inhibition of alpha-keto esters towards cathepsin B at pH 5.2More data for this Ligand-Target Pair
Affinity DataKi: 1.50E+3nMpH: 5.2Assay Description:Inhibition of alpha-keto esters towards cathepsin B at pH 5.2More data for this Ligand-Target Pair
Affinity DataKi: 2.40E+3nMpH: 5.2Assay Description:Inhibition of alpha-keto esters towards cathepsin B at pH 5.2More data for this Ligand-Target Pair
Affinity DataKi: 2.60E+3nMpH: 5.2Assay Description:Inhibition of alpha-keto esters towards cathepsin B at pH 5.2More data for this Ligand-Target Pair
Affinity DataKi: 2.60E+3nMpH: 5.2Assay Description:Inhibition of alpha-keto esters towards cathepsin B at pH 5.2More data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nMpH: 5.2Assay Description:Inhibition of alpha-keto esters towards cathepsin B at pH 5.2More data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nMpH: 5.2Assay Description:Inhibition of alpha-keto esters towards cathepsin B at pH 5.2More data for this Ligand-Target Pair
Affinity DataKi: 3.00E+3nMpH: 5.2Assay Description:Inhibition of alpha-keto esters towards cathepsin B at pH 5.2More data for this Ligand-Target Pair
Affinity DataKi: 3.30E+3nMpH: 5.2Assay Description:Inhibition of alpha-keto esters towards cathepsin B at pH 5.2More data for this Ligand-Target Pair
Affinity DataKi: 3.40E+3nMpH: 5.2Assay Description:Inhibition of alpha-keto esters towards cathepsin B at pH 5.2More data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMpH: 5.2Assay Description:Inhibition of alpha-keto esters towards cathepsin B at pH 5.2More data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMpH: 5.2Assay Description:Inhibition of alpha-keto esters towards cathepsin B at pH 5.2More data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMpH: 5.2Assay Description:Inhibition of alpha-keto esters towards cathepsin B at pH 5.2More data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMpH: 5.2Assay Description:Inhibition of alpha-keto esters towards cathepsin B at pH 5.2More data for this Ligand-Target Pair