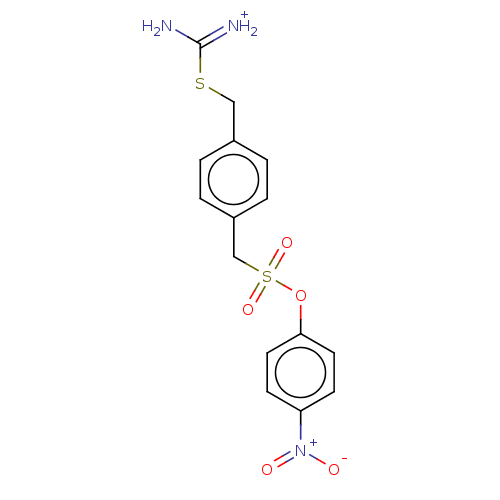
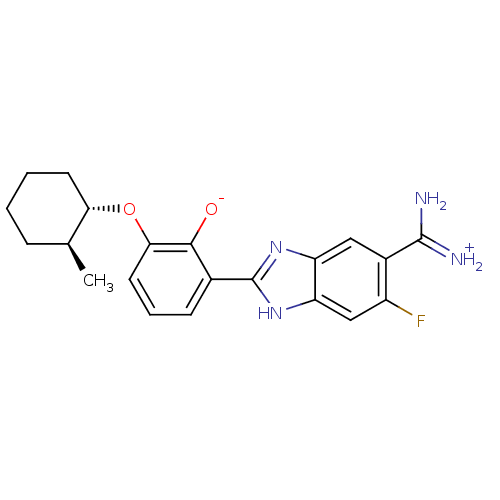
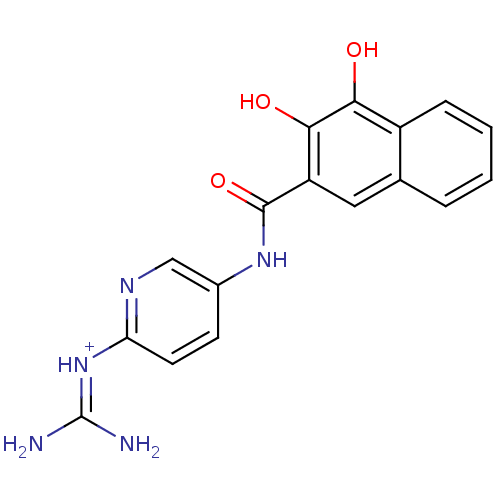
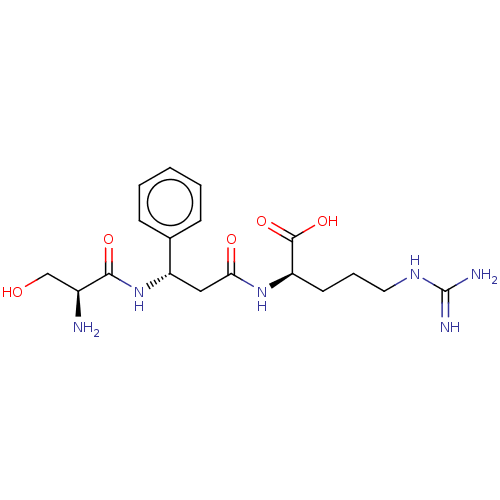
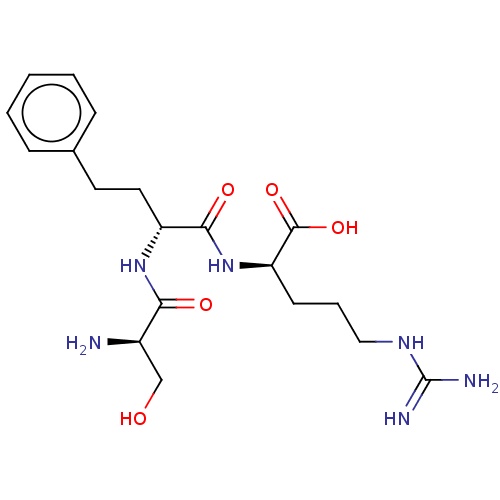
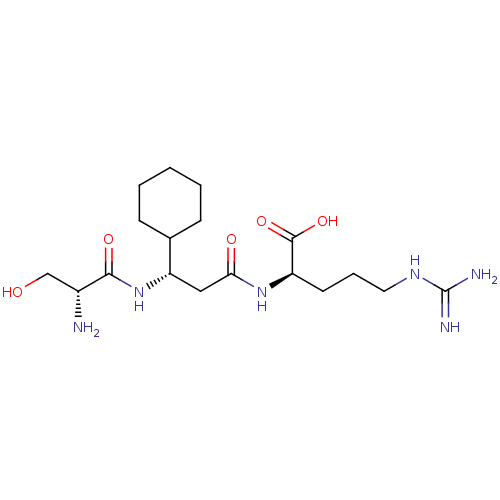
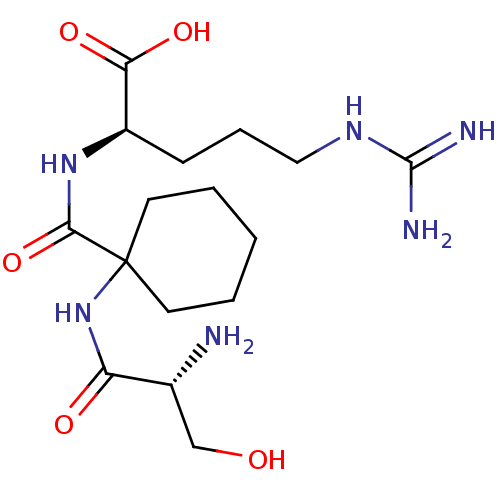
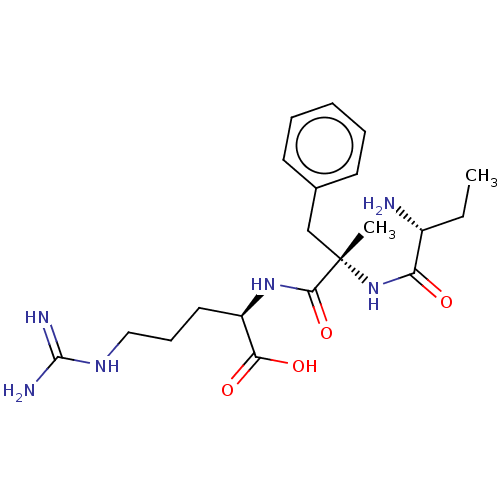
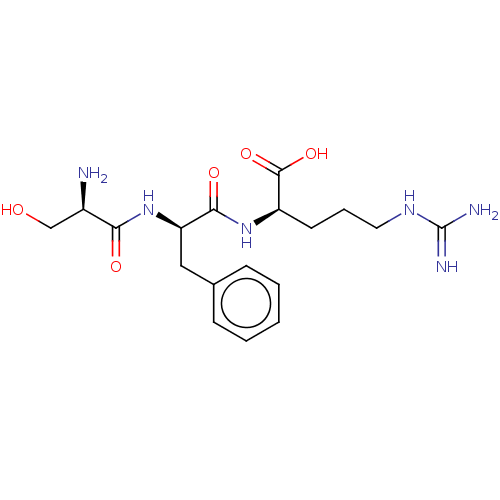
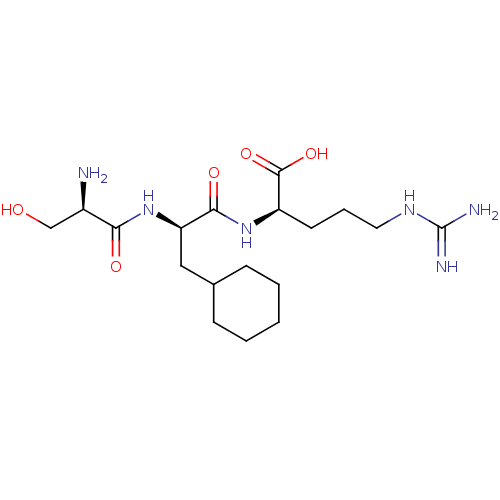
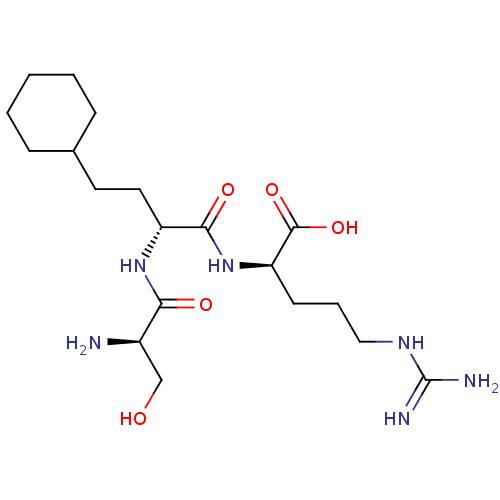
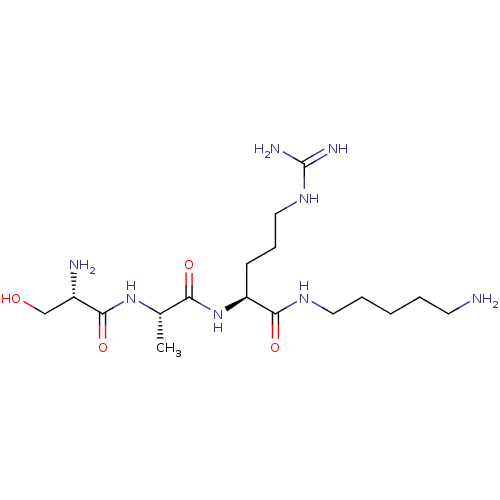
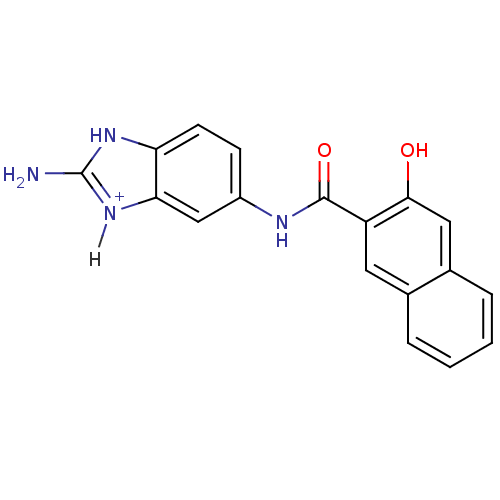
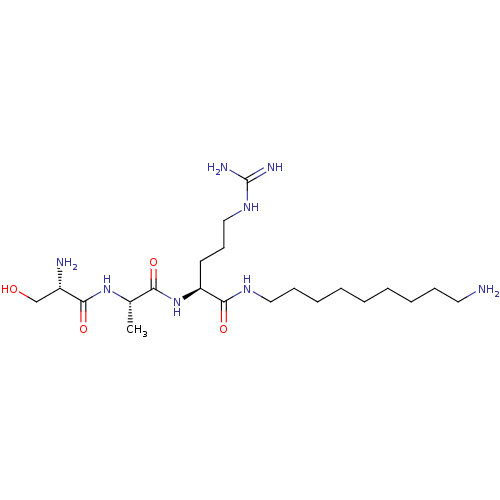
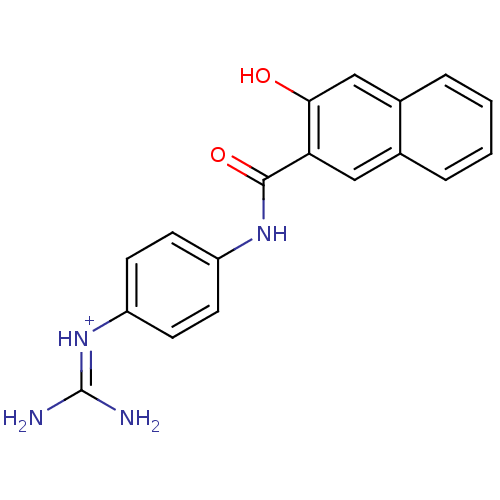
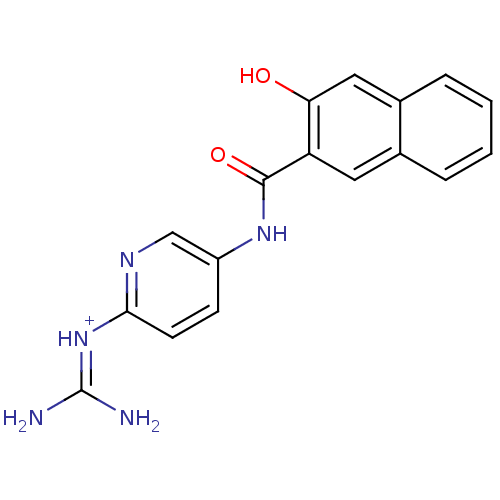
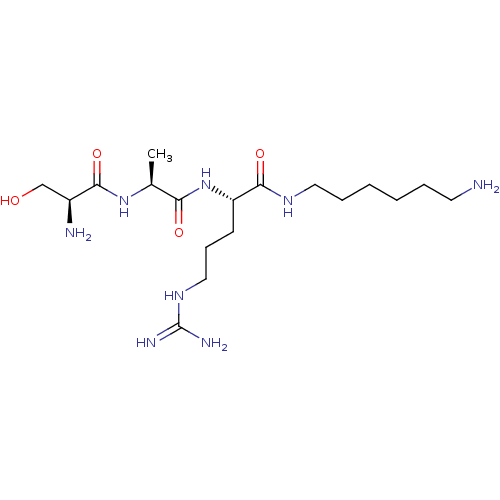
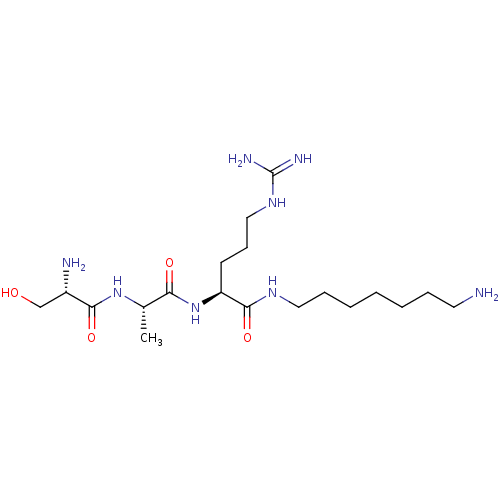
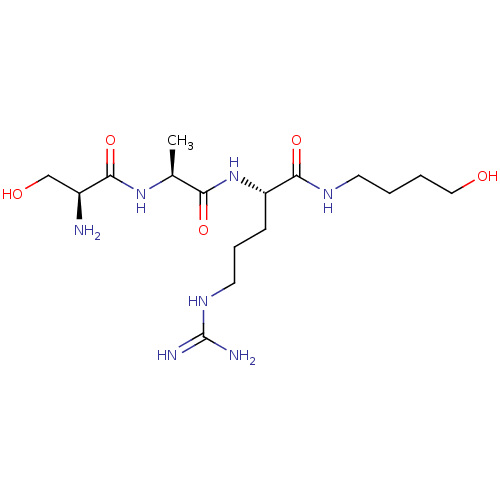
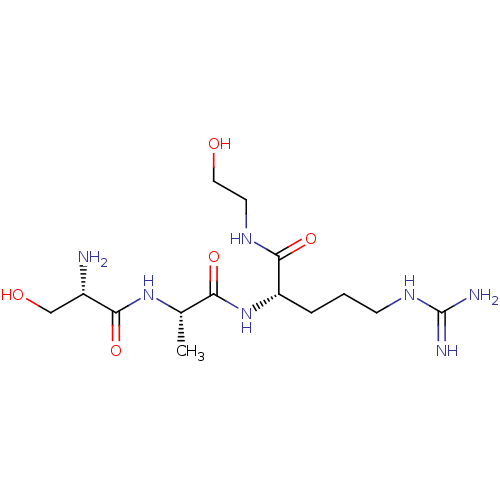
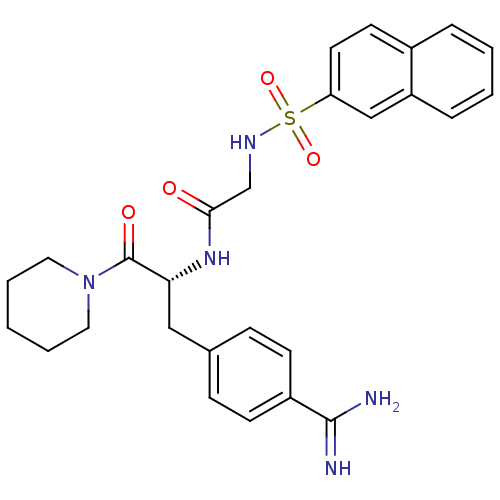
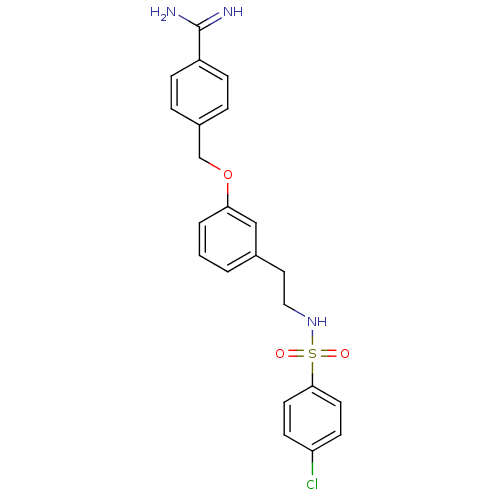
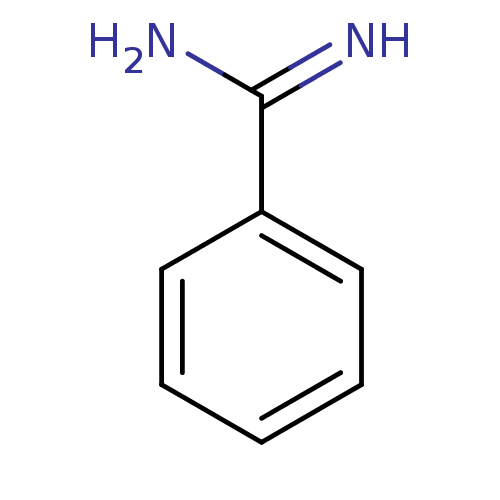
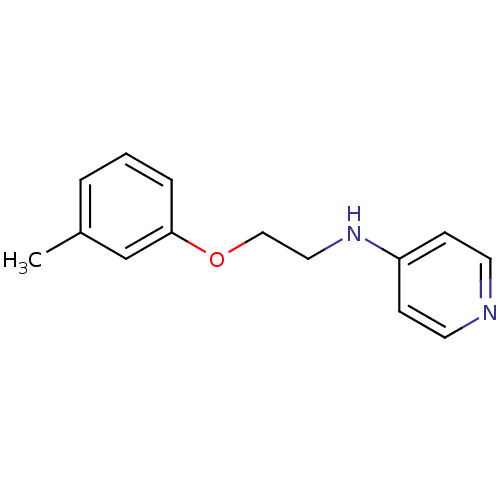
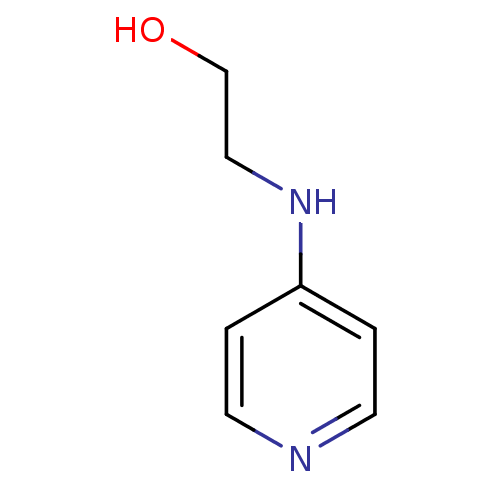
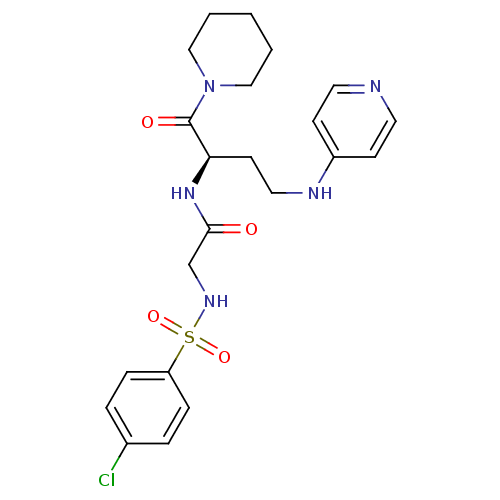
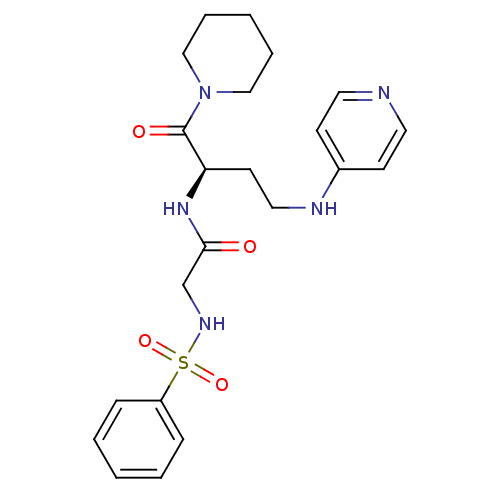
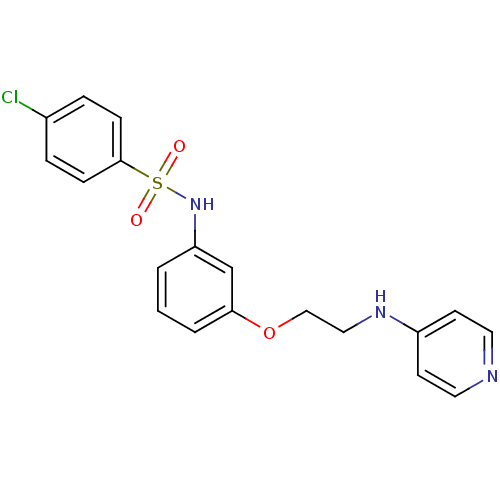
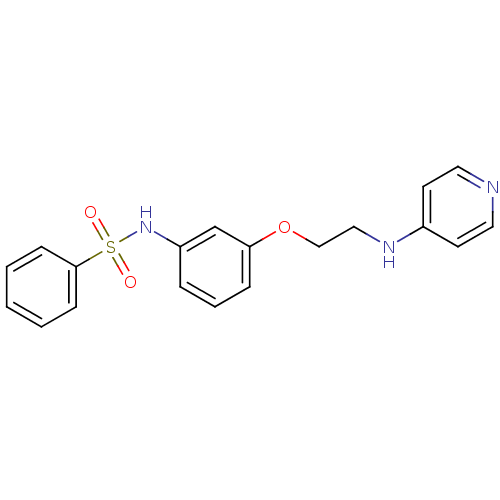
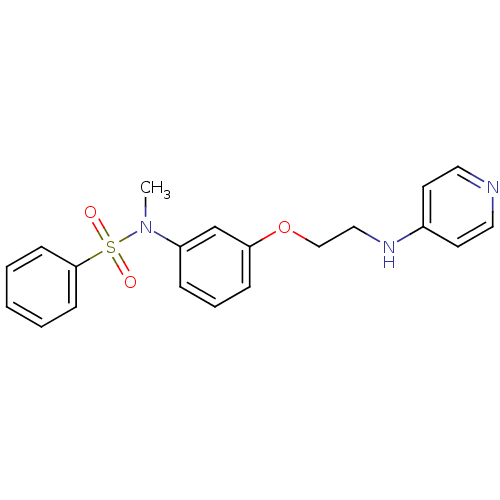
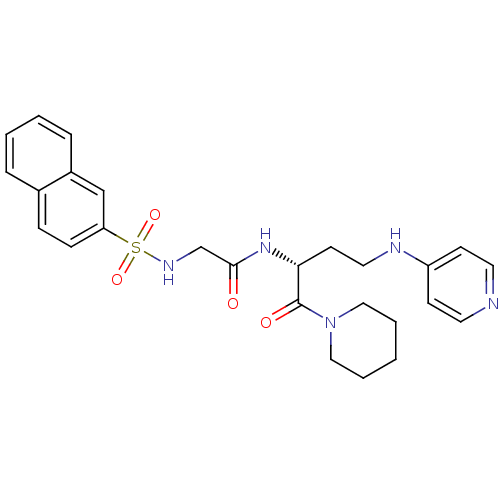
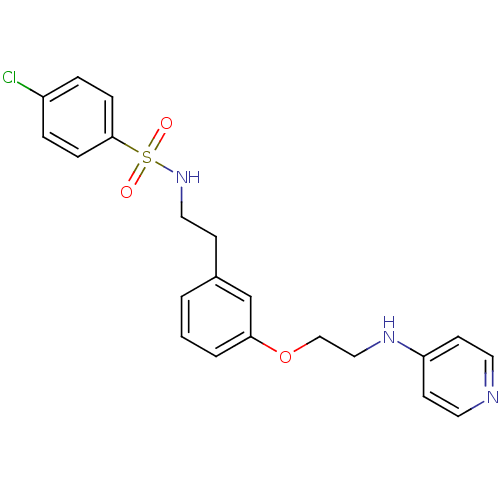
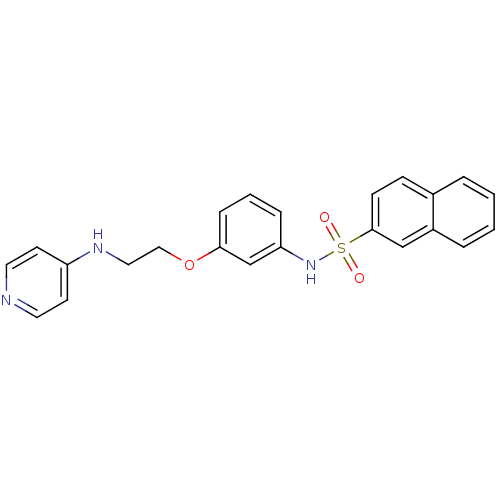
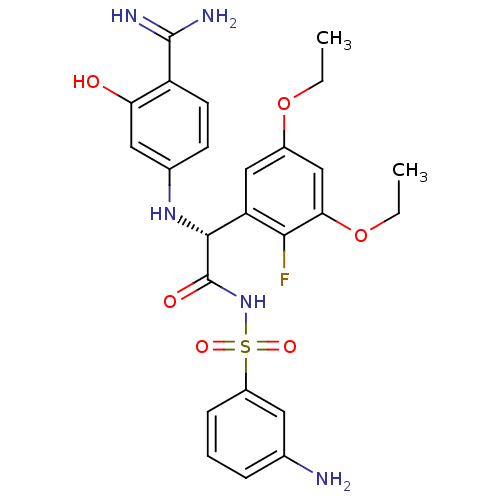
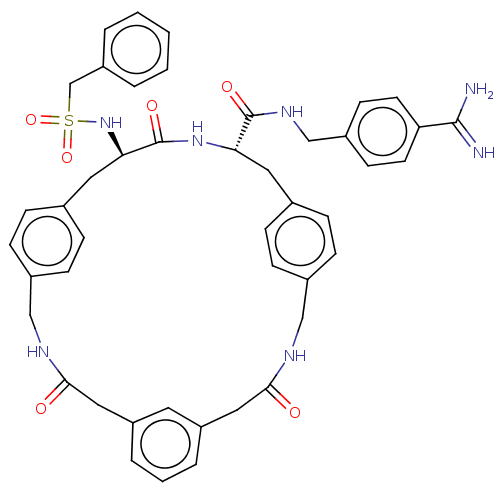
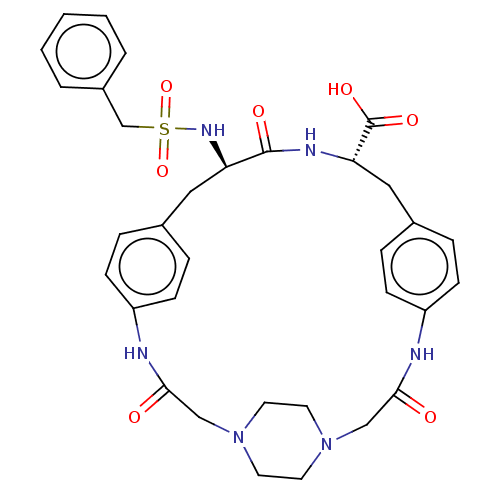
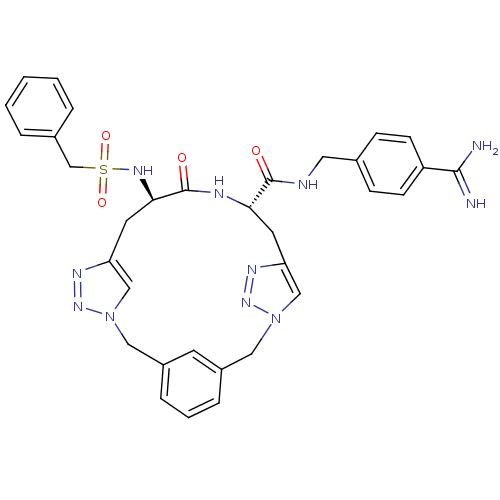
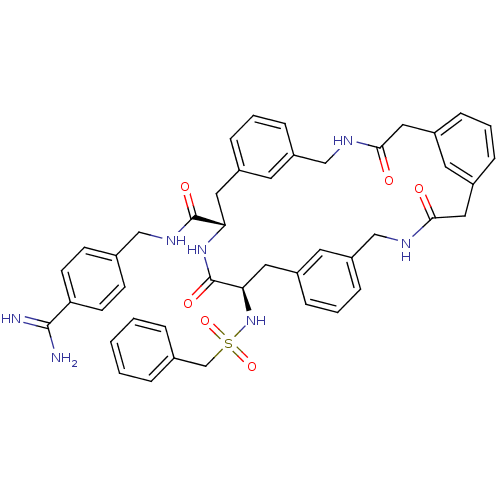
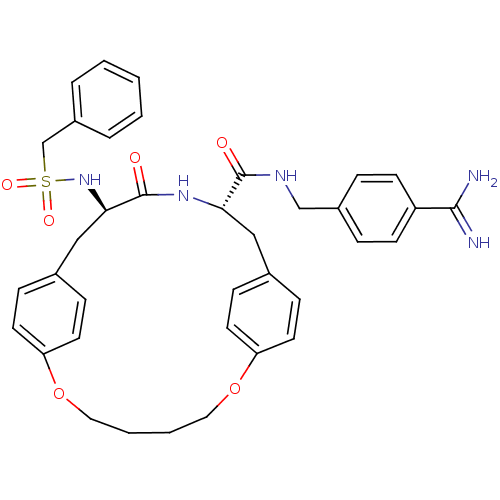
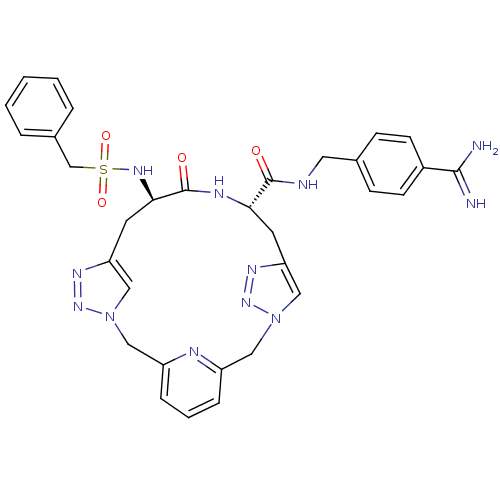
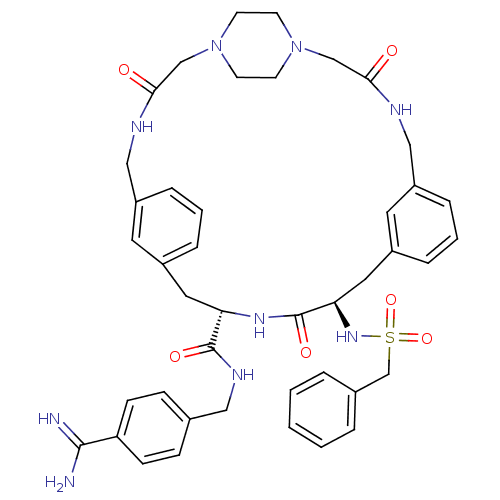
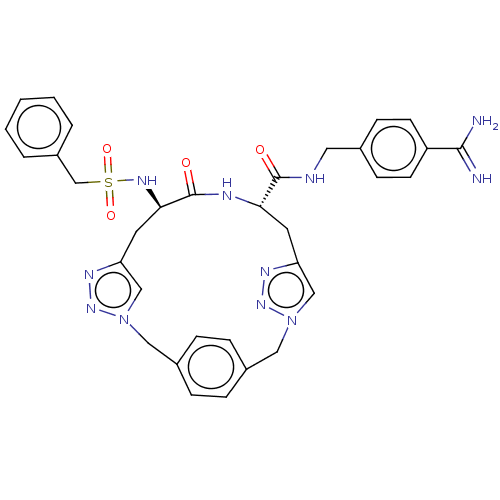
Affinity DataKi: 1.20E+6nMpH: 6.0Assay Description:Competitive inhibition of 4.8 X 10'-7 M human plasmin using Z-Lys-nitrophenyl ester as substrate incubated up to 1 hr at pH 6More data for this Ligand-Target Pair
Affinity DataKi: 8.20nMpH: 7.4Assay Description:Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en...More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nM ΔG°: -33.7kJ/molepH: 7.4 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nM ΔG°: -30.5kJ/molepH: 7.4 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 5.70E+3nMpH: 7.4Assay Description:Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en...More data for this Ligand-Target Pair
Affinity DataKi: 5.90E+3nMpH: 7.4Assay Description:Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en...More data for this Ligand-Target Pair
Affinity DataKi: 6.40E+3nM ΔG°: -29.3kJ/molepH: 7.4 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 7.70E+3nMpH: 7.4Assay Description:Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en...More data for this Ligand-Target Pair
Affinity DataKi: 7.70E+3nMpH: 7.4Assay Description:Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en...More data for this Ligand-Target Pair
Affinity DataKi: 7.90E+3nMpH: 7.4Assay Description:Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en...More data for this Ligand-Target Pair
Affinity DataKi: 8.70E+3nMpH: 7.4Assay Description:Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en...More data for this Ligand-Target Pair
Affinity DataKi: 8.90E+3nMpH: 7.4Assay Description:Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en...More data for this Ligand-Target Pair
Affinity DataKi: 1.12E+4nMpH: 7.4Assay Description:Buffer and 0.1 mL of enzyme solution was added to 0.2 mL of examined compounddissolved in 0.15 M NaCl (as control 0.15 M NaCl). The buffer and the en...More data for this Ligand-Target Pair
Affinity DataKi: 3.70E+4nMpH: 7.4Assay Description:Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ...More data for this Ligand-Target Pair
Affinity DataKi: >7.50E+4nM ΔG°: >-23.3kJ/molepH: 7.4 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: >7.50E+4nM ΔG°: >-23.3kJ/molepH: 7.4 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 9.00E+4nMpH: 7.4Assay Description:Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ...More data for this Ligand-Target Pair
Affinity DataKi: 1.30E+5nM ΔG°: -22.0kJ/molepH: 7.4 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 1.30E+5nM ΔG°: -22.0kJ/molepH: 7.4 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 1.81E+5nMpH: 7.4Assay Description:Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ...More data for this Ligand-Target Pair
Affinity DataKi: 3.64E+5nMpH: 7.4Assay Description:Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ...More data for this Ligand-Target Pair
Affinity DataKi: 5.45E+5nMpH: 7.4Assay Description:Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ...More data for this Ligand-Target Pair
Affinity DataKi: 7.27E+5nMpH: 7.4Assay Description:Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ...More data for this Ligand-Target Pair
Affinity DataKi: 1.09E+6nMpH: 7.4Assay Description:Determination of amidolytic activity was performed as described previously [Okada et al., Chem. Pharm. Bull., 36:1289-1297]. Detailed description of ...More data for this Ligand-Target Pair
Affinity DataKi: 3.00E+4nMpH: 7.5Assay Description:Tested for the Inhibition of the catalytic activity of human plasmin at 25 degree C and pH 7.5.More data for this Ligand-Target Pair
Affinity DataKi: 3.40E+4nMpH: 7.5Assay Description:Tested for the Inhibition of the catalytic activity of human plasmin at 25 degree C and pH 7.5.More data for this Ligand-Target Pair
Affinity DataKi: 3.50E+5nMpH: 7.5Assay Description:Tested for the Inhibition of the catalytic activity of human plasmin at 25 degree C and pH 7.5.More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+5nMpH: 7.5Assay Description:Tested for the Inhibition of the catalytic activity of human plasmin at 25 degree C and pH 7.5.More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+5nMpH: 7.5Assay Description:Tested for the Inhibition of the catalytic activity of human plasmin at 25 degree C and pH 7.5.More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+5nMpH: 7.5Assay Description:Tested for the Inhibition of the catalytic activity of human plasmin at 25 degree C and pH 7.5.More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+5nMpH: 7.5Assay Description:Tested for the Inhibition of the catalytic activity of human plasmin at 25 degree C and pH 7.5.More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+5nMpH: 7.5Assay Description:Tested for the Inhibition of the catalytic activity of human plasmin at 25 degree C and pH 7.5.More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+5nMpH: 7.5Assay Description:Tested for the Inhibition of the catalytic activity of human plasmin at 25 degree C and pH 7.5.More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+5nMpH: 7.5Assay Description:Tested for the Inhibition of the catalytic activity of human plasmin at 25 degree C and pH 7.5.More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+5nMpH: 7.5Assay Description:Tested for the Inhibition of the catalytic activity of human plasmin at 25 degree C and pH 7.5.More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+5nMpH: 7.5Assay Description:Tested for the Inhibition of the catalytic activity of human plasmin at 25 degree C and pH 7.5.More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+5nMpH: 7.5Assay Description:Tested for the Inhibition of the catalytic activity of human plasmin at 25 degree C and pH 7.5.More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+5nMpH: 7.5Assay Description:Tested for the Inhibition of the catalytic activity of human plasmin at 25 degree C and pH 7.5.More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+5nMpH: 7.5Assay Description:Tested for the Inhibition of the catalytic activity of human plasmin at 25 degree C and pH 7.5.More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+6nMpH: 7.5Assay Description:Tested for the Inhibition of the catalytic activity of human plasmin at 25 degree C and pH 7.5.More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nM ΔG°: -33.5kJ/molepH: 7.8 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 0.550nMpH: 8.0Assay Description:The inhibition constants for human plasmin (h plasmin), human plasma kallikrein (h PK), thrombin and factor Xa were determined in analogy to a previo...More data for this Ligand-Target Pair
Affinity DataKi: 0.680nMpH: 8.0Assay Description:The inhibition constants for human plasmin (h plasmin), human plasma kallikrein (h PK), thrombin and factor Xa were determined in analogy to a previo...More data for this Ligand-Target Pair
Affinity DataKi: 0.770nMpH: 8.0Assay Description:The inhibition constants for human plasmin (h plasmin), human plasma kallikrein (h PK), thrombin and factor Xa were determined in analogy to a previo...More data for this Ligand-Target Pair
Affinity DataKi: 1.10nMpH: 8.0Assay Description:The inhibition constants for human plasmin (h plasmin), human plasma kallikrein (h PK), thrombin and factor Xa were determined in analogy to a previo...More data for this Ligand-Target Pair
Affinity DataKi: 1.90nMpH: 8.0Assay Description:The inhibition constants for human plasmin (h plasmin), human plasma kallikrein (h PK), thrombin and factor Xa were determined in analogy to a previo...More data for this Ligand-Target Pair
Affinity DataKi: 2.20nMpH: 8.0Assay Description:The inhibition constants for human plasmin (h plasmin), human plasma kallikrein (h PK), thrombin and factor Xa were determined in analogy to a previo...More data for this Ligand-Target Pair
Affinity DataKi: 2.5nMpH: 8.0Assay Description:The inhibition constants for human plasmin (h plasmin), human plasma kallikrein (h PK), thrombin and factor Xa were determined in analogy to a previo...More data for this Ligand-Target Pair
Affinity DataKi: 4.90nMpH: 8.0Assay Description:The inhibition constants for human plasmin (h plasmin), human plasma kallikrein (h PK), thrombin and factor Xa were determined in analogy to a previo...More data for this Ligand-Target Pair
Affinity DataKi: 5.40nMpH: 8.0Assay Description:The inhibition constants for human plasmin (h plasmin), human plasma kallikrein (h PK), thrombin and factor Xa were determined in analogy to a previo...More data for this Ligand-Target Pair