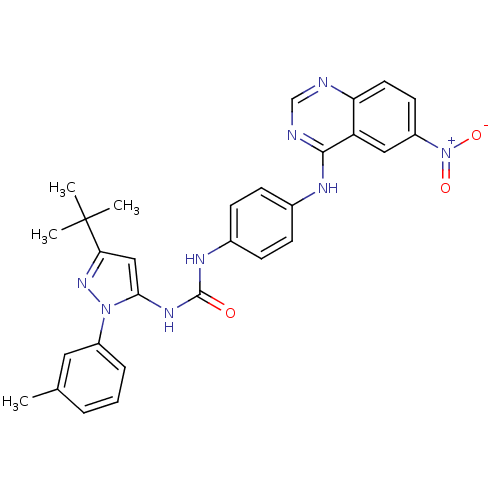
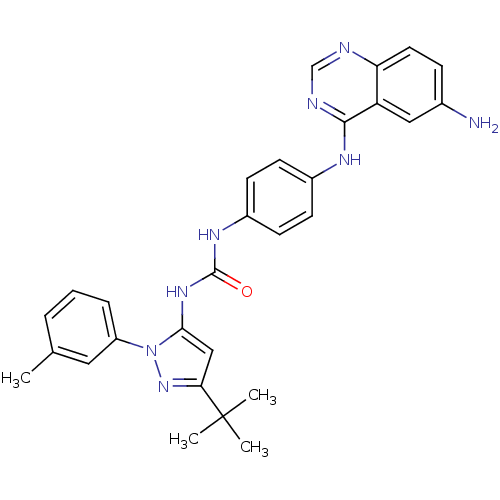
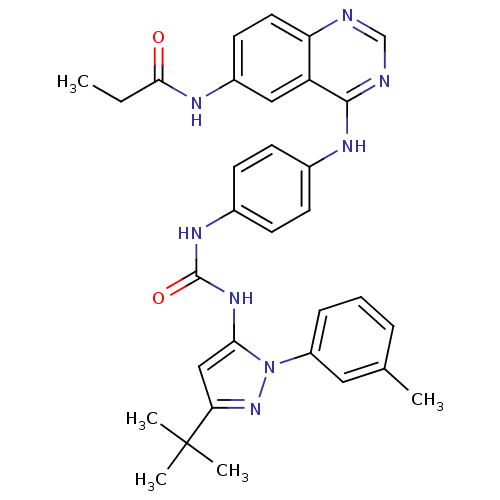
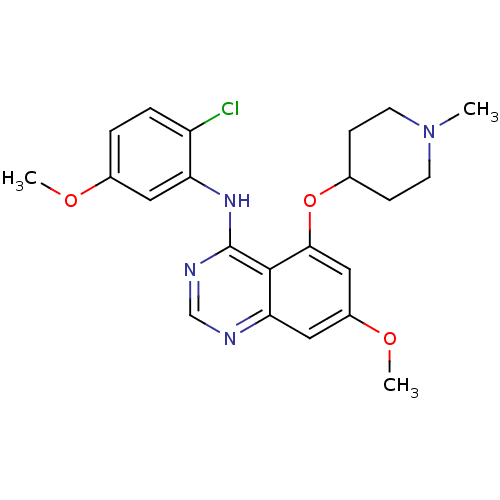
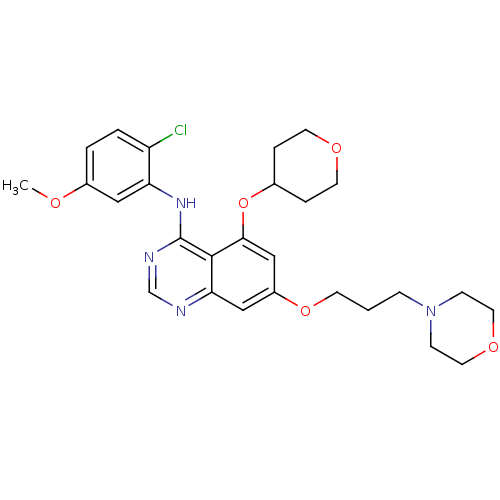
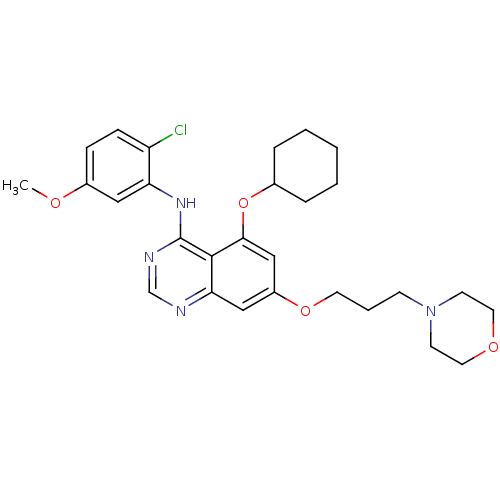
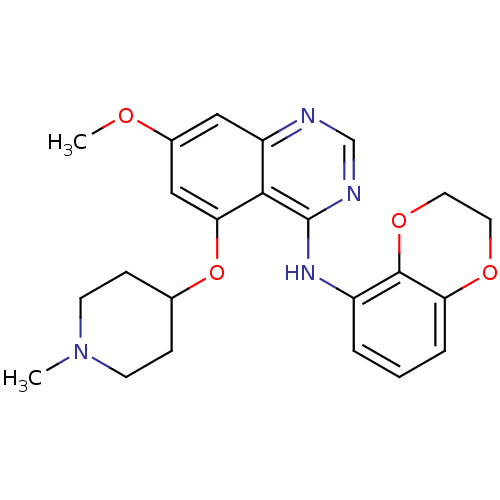
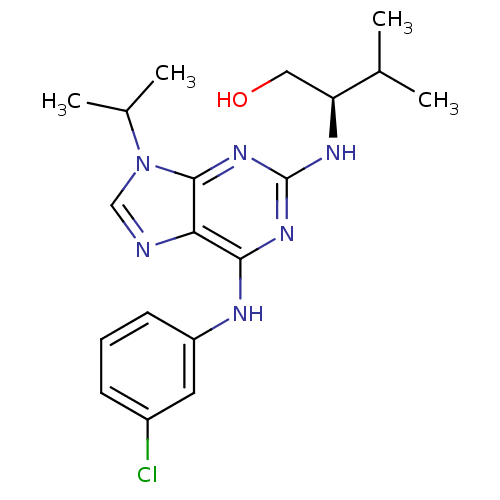
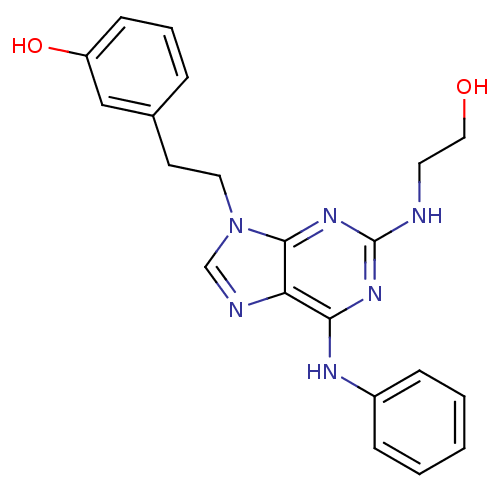
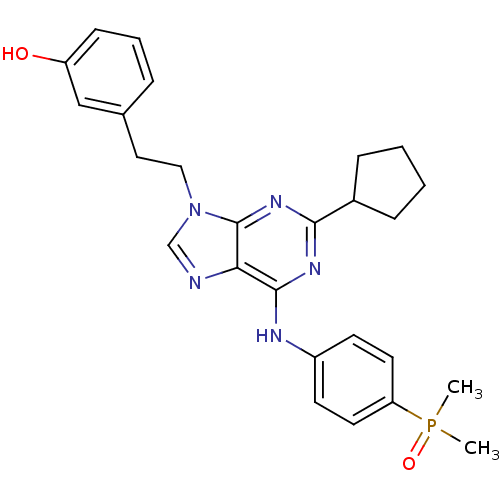
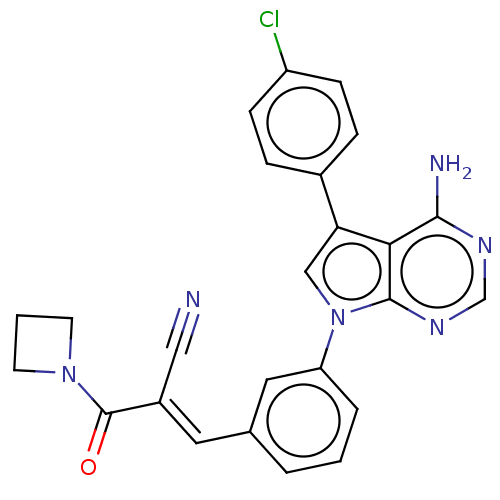
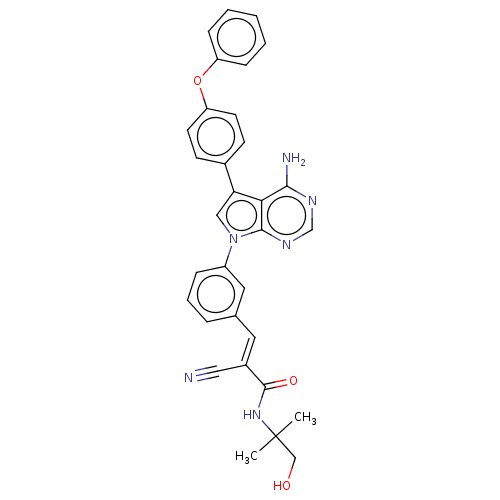
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: >5.00E+3nMpH: 7.0 T: 2°CAssay Description:Src, Lck, Flt-1, ZAP, EGFR, FGFR1, and PFGFR-beta were assayed in the Merck research laboratory (Homogeneous proximity tyrosine kinase assays: scinti...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Gallus gallus (Chicken))
Chemical Genomics Centre Of The Max Planck Society
Chemical Genomics Centre Of The Max Planck Society
Affinity DataIC50: 3.21E+4nMpH: 7.0 T: 2°CAssay Description:IC50 determinations for cSrc kinases were measured with the HTRF KinEASE-TK assay from Cisbio according to the manufacturer instructions. A biotinyla...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Gallus gallus (Chicken))
Chemical Genomics Centre Of The Max Planck Society
Chemical Genomics Centre Of The Max Planck Society
Affinity DataIC50: 6.41E+4nMpH: 7.0 T: 2°CAssay Description:IC50 determinations for cSrc kinases were measured with the HTRF KinEASE-TK assay from Cisbio according to the manufacturer instructions. A biotinyla...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Gallus gallus (Chicken))
Chemical Genomics Centre Of The Max Planck Society
Chemical Genomics Centre Of The Max Planck Society
Affinity DataIC50: 6.40E+3nMpH: 7.0 T: 2°CAssay Description:IC50 determinations for cSrc kinases were measured with the HTRF KinEASE-TK assay from Cisbio according to the manufacturer instructions. A biotinyla...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Gallus gallus (Chicken))
Chemical Genomics Centre Of The Max Planck Society
Chemical Genomics Centre Of The Max Planck Society
Affinity DataIC50: 235nM Kd: 239nMpH: 7.0 T: 2°CAssay Description:IC50 determinations for cSrc kinases were measured with the HTRF KinEASE-TK assay from Cisbio according to the manufacturer instructions. A biotinyla...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Gallus gallus (Chicken))
Chemical Genomics Centre Of The Max Planck Society
Chemical Genomics Centre Of The Max Planck Society
Affinity DataIC50: 71nM Kd: 174nMpH: 7.0 T: 2°CAssay Description:IC50 determinations for cSrc kinases were measured with the HTRF KinEASE-TK assay from Cisbio according to the manufacturer instructions. A biotinyla...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Gallus gallus (Chicken))
Chemical Genomics Centre Of The Max Planck Society
Chemical Genomics Centre Of The Max Planck Society
Affinity DataIC50: 21nM Kd: 73nMpH: 7.0 T: 2°CAssay Description:IC50 determinations for cSrc kinases were measured with the HTRF KinEASE-TK assay from Cisbio according to the manufacturer instructions. A biotinyla...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Gallus gallus (Chicken))
Chemical Genomics Centre Of The Max Planck Society
Chemical Genomics Centre Of The Max Planck Society
Affinity DataIC50: 14nM Kd: 56nMpH: 7.0 T: 2°CAssay Description:IC50 determinations for cSrc kinases were measured with the HTRF KinEASE-TK assay from Cisbio according to the manufacturer instructions. A biotinyla...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Gallus gallus (Chicken))
Chemical Genomics Centre Of The Max Planck Society
Chemical Genomics Centre Of The Max Planck Society
Affinity DataIC50: 207nM Kd: 256nMpH: 7.0 T: 2°CAssay Description:IC50 determinations for cSrc kinases were measured with the HTRF KinEASE-TK assay from Cisbio according to the manufacturer instructions. A biotinyla...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Gallus gallus (Chicken))
Chemical Genomics Centre Of The Max Planck Society
Chemical Genomics Centre Of The Max Planck Society
Affinity DataIC50: 0.400nM Kd: 11nMpH: 7.0 T: 2°CAssay Description:IC50 determinations for cSrc kinases were measured with the HTRF KinEASE-TK assay from Cisbio according to the manufacturer instructions. A biotinyla...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 300nMpH: 7.4 T: 2°CAssay Description:This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 400nMpH: 7.4 T: 2°CAssay Description:This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 100nMpH: 7.4 T: 2°CAssay Description:This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 230nMpH: 7.4 T: 2°CAssay Description:This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 30nMpH: 7.4 T: 2°CAssay Description:This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 10nMpH: 7.4 T: 2°CAssay Description:This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 20nMpH: 7.4 T: 2°CAssay Description:This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 20nMpH: 7.4 T: 2°CAssay Description:This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: <4nMpH: 7.4 T: 2°CAssay Description:This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 40nMpH: 7.4 T: 2°CAssay Description:This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 40nMpH: 7.4 T: 2°CAssay Description:This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 150nMpH: 7.4 T: 2°CAssay Description:This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 570nMpH: 7.4 T: 2°CAssay Description:This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 80nMpH: 7.4 T: 2°CAssay Description:This assay determines the ability of test compounds to inhibit Src kinase activity that catalyzes the transfer of the terminal phosphate to the immob...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 240nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 1.59E+3nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 25nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 26nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 34nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 0.450nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 2.11E+3nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 239nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 67nMpH: 7.4 T: 2°CAssay Description:Inhibition of human Src kinase activity by ARIAD compounds were measured in a homogeneous time-resolved fluorescence resonance energy transfer (TR-FR...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 5.90E+3nMpH: 7.4 T: 2°CAssay Description:Wild-type and S345C mutant cSrc kinase domains were expressed and purified as described (Blair et al., Nat. Chem. Biol., 2007). The purified cSrc kin...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 640nMpH: 7.4 T: 2°CAssay Description:Wild-type and S345C mutant cSrc kinase domains were expressed and purified as described (Blair et al., Nat. Chem. Biol., 2007). The purified cSrc kin...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: >5.00E+3nMpH: 7.4 T: 2°CAssay Description:Wild-type and S345C mutant cSrc kinase domains were expressed and purified as described (Blair et al., Nat. Chem. Biol., 2007). The purified cSrc kin...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: >5.00E+3nMpH: 7.4 T: 2°CAssay Description:Wild-type and S345C mutant cSrc kinase domains were expressed and purified as described (Blair et al., Nat. Chem. Biol., 2007). The purified cSrc kin...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: >5.00E+3nMpH: 7.4 T: 2°CAssay Description:Wild-type and S345C mutant cSrc kinase domains were expressed and purified as described (Blair et al., Nat. Chem. Biol., 2007). The purified cSrc kin...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: >1.00E+4nMpH: 7.4 T: 2°CAssay Description:Wild-type and S345C mutant cSrc kinase domains were expressed and purified as described (Blair et al., Nat. Chem. Biol., 2007). The purified cSrc kin...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 260nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 4.20E+3nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: >5.00E+4nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: >5.00E+4nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 750nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 590nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 220nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 210nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 750nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 73nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Banyu Tsukuba Research Institute
Banyu Tsukuba Research Institute
Affinity DataIC50: 430nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair




 3D Structure (crystal)
3D Structure (crystal)