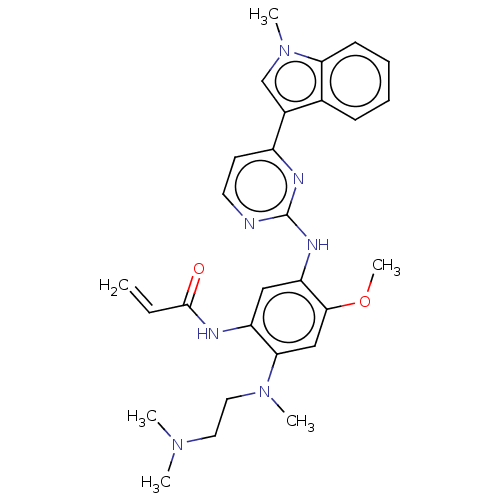
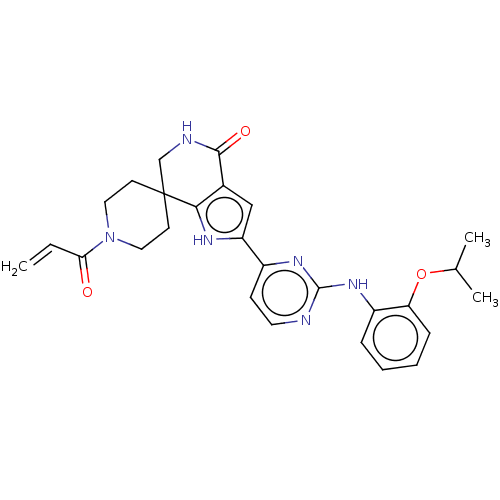
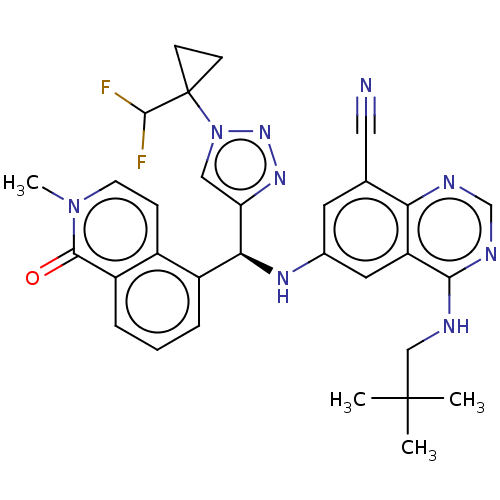
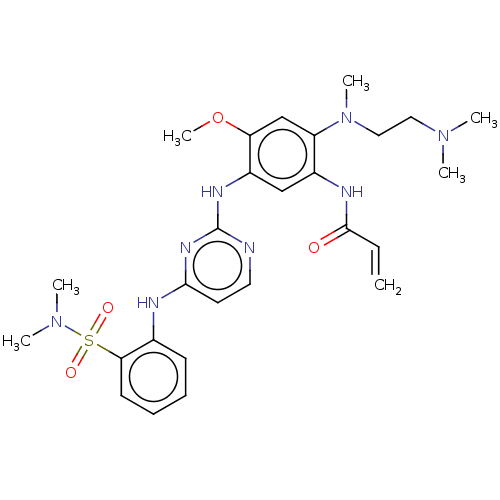
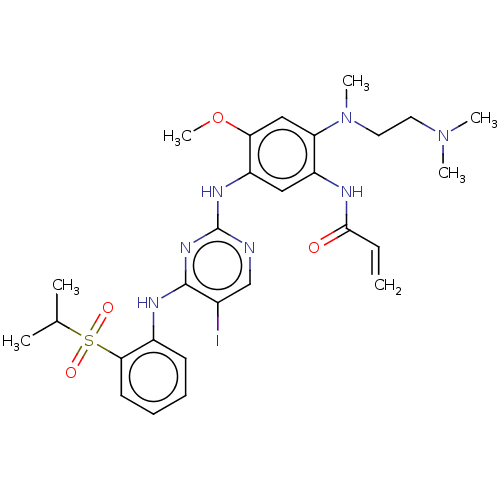
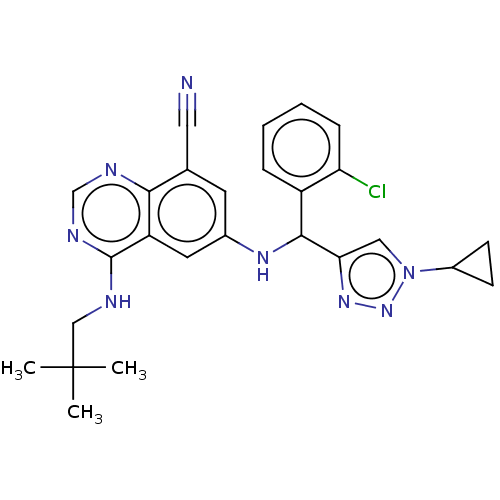
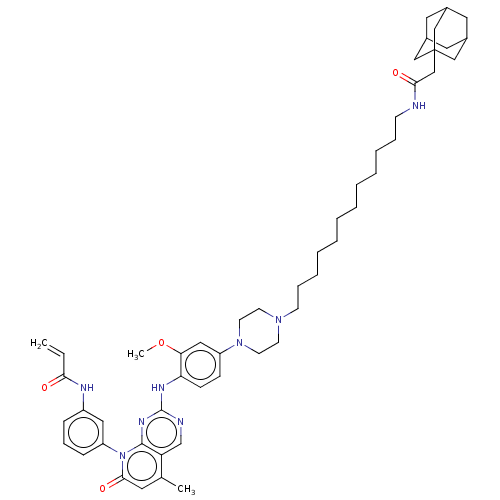
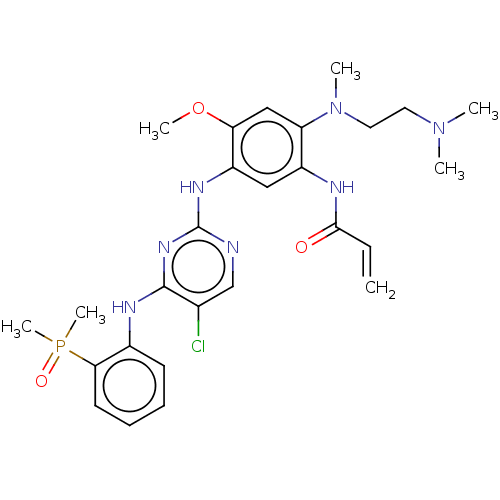
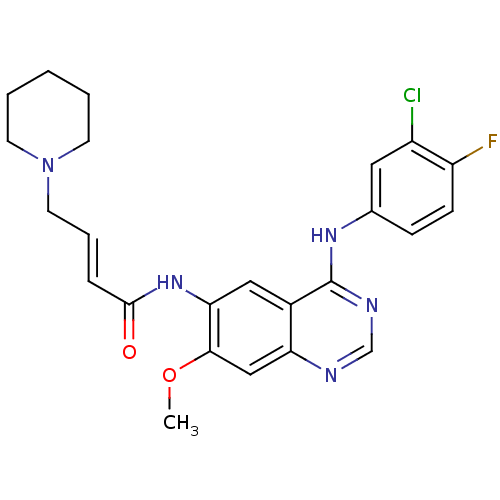
Affinity DataEC50: 2nMAssay Description:Inhibition of EGFR L858R mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by MTS assayMore data for this Ligand-Target Pair
Affinity DataEC50: 3nMAssay Description:Inhibition of EGF-stimulated EGFR phosphorylation in human A-431 cells incubated for 3 hrs followed by replacement with fresh medium without compound...More data for this Ligand-Target Pair
Affinity DataEC50: 3.10nMAssay Description:Inhibition of EGF-induced phosphorylation of EGFR T790M/L858R double mutant in human NCI-H1975 cells preincubated for 120 mins followed by EGF stimul...More data for this Ligand-Target Pair
Affinity DataEC50: 4nMAssay Description:Inhibition of EGFR L858R mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by MTS assayMore data for this Ligand-Target Pair
Affinity DataEC50: 4nMAssay Description:Inhibition of EGFR L858R mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by MTS assayMore data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Inhibition of EGFR T790M exon19 deletion double mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after...More data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Inhibition of EGFR exon19 deletion mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by MT...More data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Inhibition of EGFR T790M/L858R double mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by...More data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Inhibition of EGFR T790M exon19 deletion double mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after...More data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Inhibition of EGFR T790M exon19 deletion double mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after...More data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Inhibition of EGFR T790M exon19 deletion double mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after...More data for this Ligand-Target Pair
Affinity DataEC50: 5.80nMAssay Description:Inhibition of wild type human EGFR expressed in Sf9 insect cells in presence of ATP measured by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 6nMAssay Description:Inhibition of EGFR T790M/L858R double mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by...More data for this Ligand-Target Pair
Affinity DataEC50: 6nMAssay Description:Inhibition of EGFR T790M/L858R double mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by...More data for this Ligand-Target Pair
Affinity DataEC50: 6nMAssay Description:Inhibition of EGFR L858R mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by MTS assayMore data for this Ligand-Target Pair
Affinity DataEC50: 6nMAssay Description:Inhibition of EGFR exon19 deletion mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by MT...More data for this Ligand-Target Pair
Affinity DataEC50: 6nMAssay Description:Inhibition of EGFR exon19 deletion mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by MT...More data for this Ligand-Target Pair
Affinity DataEC50: 6nMAssay Description:Inhibition of EGFR T790M/L858R double mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by...More data for this Ligand-Target Pair
Affinity DataEC50: 6nMAssay Description:Inhibition of EGFR exon19 deletion mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by MT...More data for this Ligand-Target Pair
Affinity DataEC50: 7.10nMAssay Description:Inhibition of EGFR T790M/L858R mutant in human NCI-H1975 cells assessed as reduction in EGFR Y1068 phosphorylation incubated for 4 hrs by Western blo...More data for this Ligand-Target Pair
Affinity DataEC50: 7.5nMAssay Description:Inhibition of EGF-induced phosphorylation of wild type EGFR in human NCI-H1975 cells preincubated for 120 mins followed by EGF stimulation and measur...More data for this Ligand-Target Pair
Affinity DataEC50: 8nMAssay Description:Inhibition of EGFR L858R mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by MTS assayMore data for this Ligand-Target Pair
Affinity DataEC50: 8.80nMAssay Description:Inhibition of EGF-induced phosphorylation of EGFR T790M/L858R double mutant in human NCI-H1975 cells preincubated for 120 mins followed by EGF stimul...More data for this Ligand-Target Pair
Affinity DataEC50: 11.3nMpH: 7.0 T: 2°CAssay Description:EGFR activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, EGFR-...More data for this Ligand-Target Pair
Affinity DataEC50: 11.3nMAssay Description:EGFR activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, EGFR-...More data for this Ligand-Target Pair
Affinity DataEC50: 12nMAssay Description:Inhibition of EGF-induced phosphorylation of EGFR T790M/L858R double mutant in human NCI-H1975 cells preincubated for 120 mins followed by EGF stimul...More data for this Ligand-Target Pair
Affinity DataEC50: 12.1nMpH: 7.0 T: 2°CAssay Description:EGFR activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, EGFR-...More data for this Ligand-Target Pair
Affinity DataEC50: 12.1nMAssay Description:EGFR activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, EGFR-...More data for this Ligand-Target Pair
Affinity DataEC50: 15nMAssay Description:Inhibition of EGFR T790M mutant phosphorylation in human NCI-H1975 cells incubated for 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataEC50: 16nMAssay Description:Inhibition of EGFR T790M exon19 deletion double mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after...More data for this Ligand-Target Pair
Affinity DataEC50: 17nMAssay Description:Inhibition of EGFR T790M exon19 deletion double mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after...More data for this Ligand-Target Pair
Affinity DataEC50: 18nMAssay Description:Inhibition of EGFR exon19 deletion mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by MT...More data for this Ligand-Target Pair
Affinity DataEC50: 19nMAssay Description:Inhibition of EGFR T790M/L858R double mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by...More data for this Ligand-Target Pair
Affinity DataEC50: 19nMAssay Description:Inhibition of EGFR L858R mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by MTS assayMore data for this Ligand-Target Pair
Affinity DataEC50: 19nMAssay Description:Inhibition of EGFR L858R mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by MTS assayMore data for this Ligand-Target Pair
Affinity DataEC50: 19nMAssay Description:Inhibition of EGFR L858R mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by MTS assayMore data for this Ligand-Target Pair
Affinity DataEC50: 19nMAssay Description:Inhibition of EGFR T790M exon19 deletion double mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after...More data for this Ligand-Target Pair
Affinity DataEC50: 20nMpH: 7.0 T: 2°CAssay Description:EGFR activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, EGFR-...More data for this Ligand-Target Pair
Affinity DataEC50: 20nMAssay Description:EGFR activity was measured using KinEASE (Cisbio), a time-resolved fluorescence resonance energy transfer (TR-FRET) immunoassay. In this assay, EGFR-...More data for this Ligand-Target Pair
Affinity DataEC50: 20nMAssay Description:Inhibition of EGFR T790M exon19 deletion double mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after...More data for this Ligand-Target Pair
Affinity DataEC50: 20nMAssay Description:Inhibition of EGFR T790M mutant in human NCI-H1975 cells incubated for 24 hrs under serum free medium by Western blot analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 20nMAssay Description:Inhibition of EGFR T790M/L858R double mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by...More data for this Ligand-Target Pair
Affinity DataEC50: 21nMAssay Description:Inhibition of EGFR exon19 deletion mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by MT...More data for this Ligand-Target Pair
Affinity DataEC50: 21nMAssay Description:Inhibition of EGFR L858R mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by MTS assayMore data for this Ligand-Target Pair
Affinity DataEC50: 22nMAssay Description:Inhibition of EGF-stimulated wild-type EGFR phosphorylation in human A-431 cells incubated for 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataEC50: 22nMAssay Description:Inhibition of EGFR T790M mutant phosphorylation in human NCI-H1975 cells incubated for 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataEC50: 22nMAssay Description:Inhibition of EGFR T790M/L858R double mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by...More data for this Ligand-Target Pair
Affinity DataEC50: 22nMAssay Description:Inhibition of EGFR T790M/L858R double mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by...More data for this Ligand-Target Pair
Affinity DataEC50: 23nMAssay Description:Inhibition of EGFR L858R mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by MTS assayMore data for this Ligand-Target Pair
Affinity DataEC50: 24nMAssay Description:Inhibition of EGFR exon19 deletion mutant (unknown origin) transfected in mouse BAF3 cells assessed as reduction in cell viability after 72 hrs by MT...More data for this Ligand-Target Pair

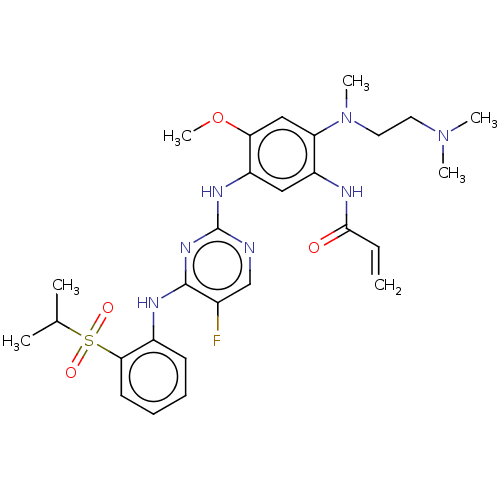
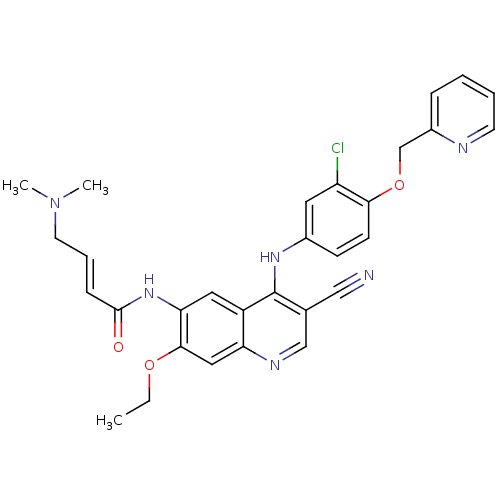

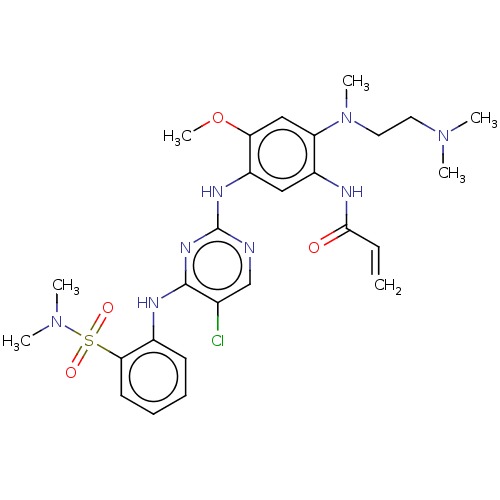
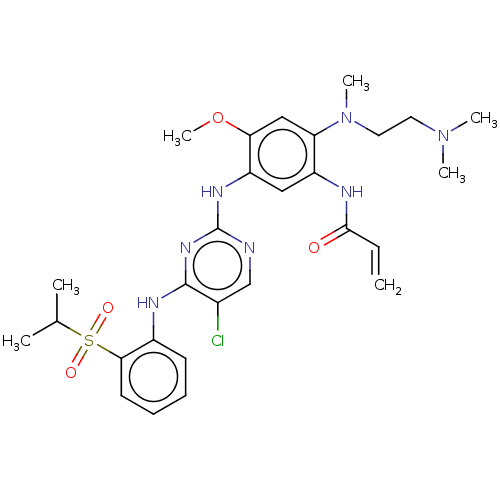
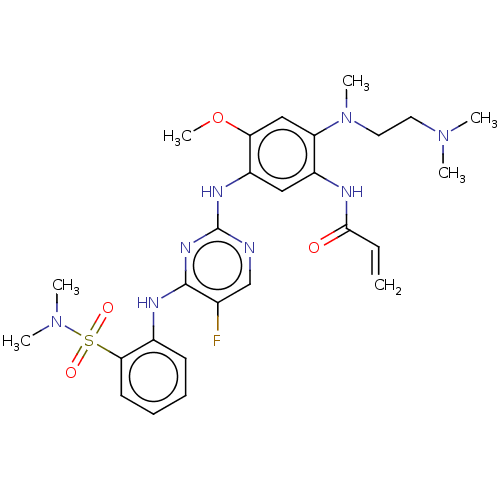
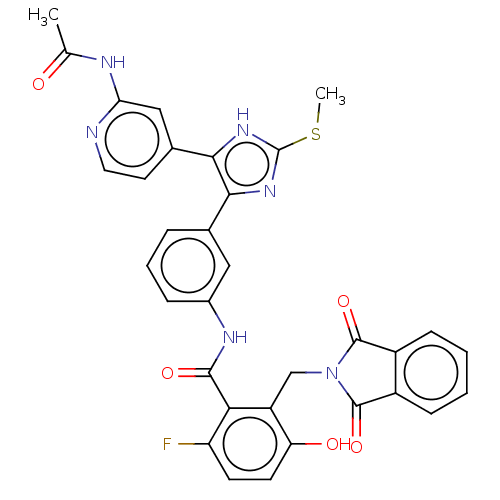
 3D Structure (crystal)
3D Structure (crystal)