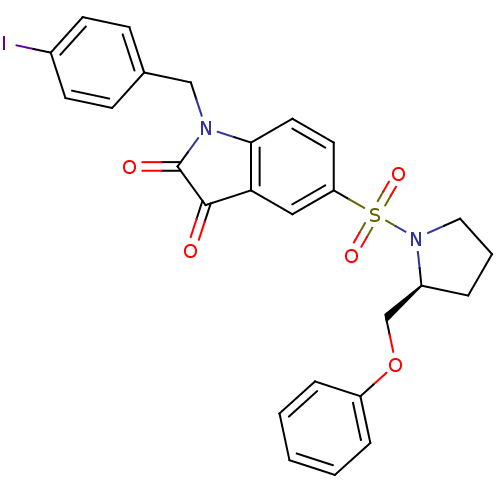
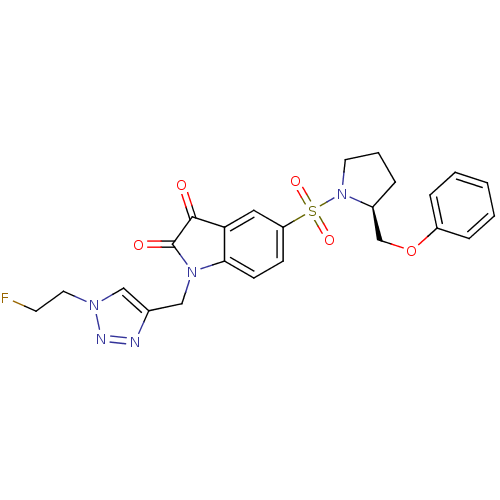
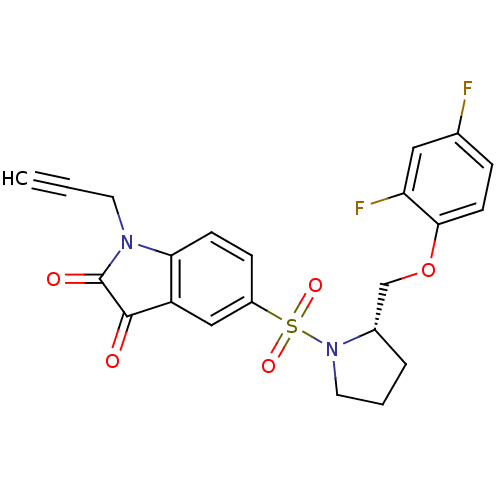
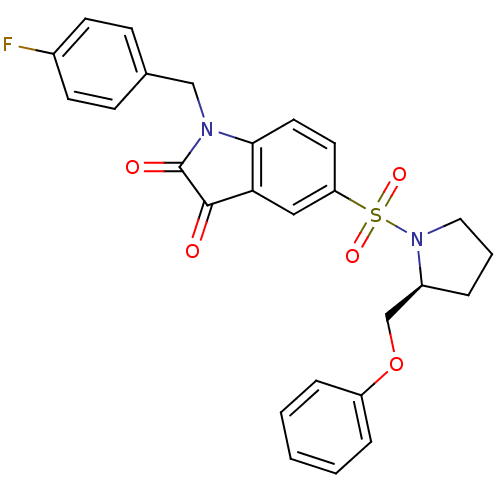
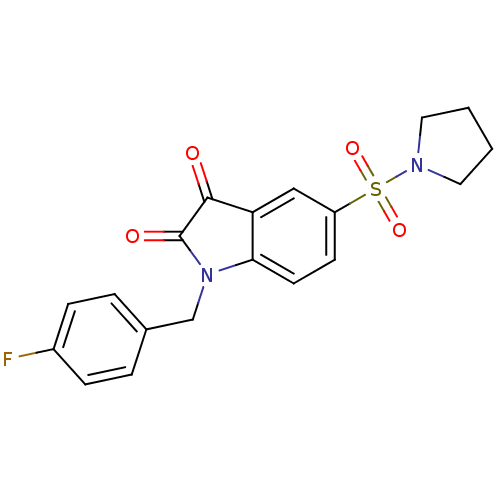
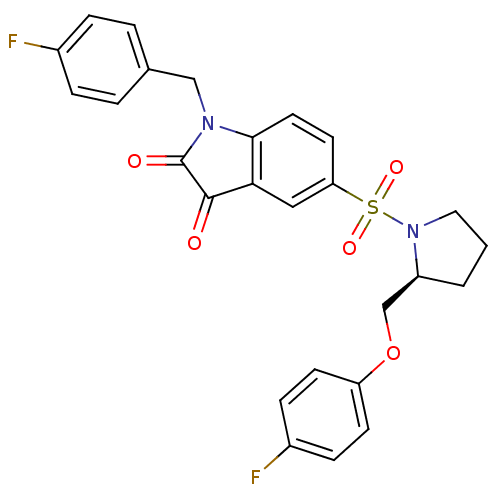
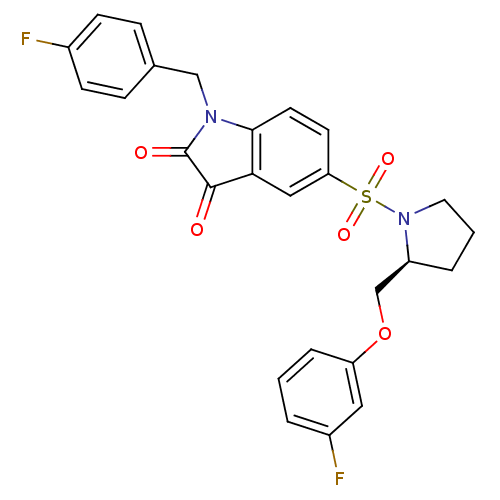
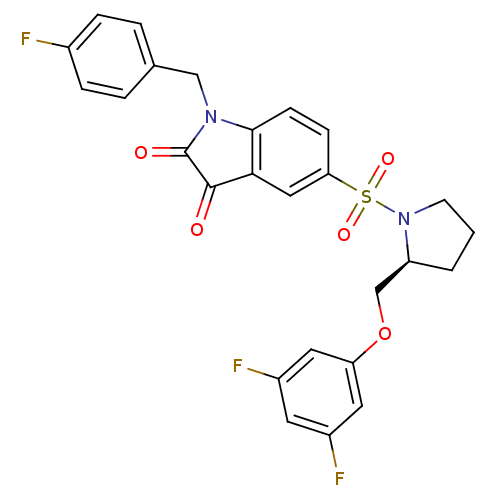
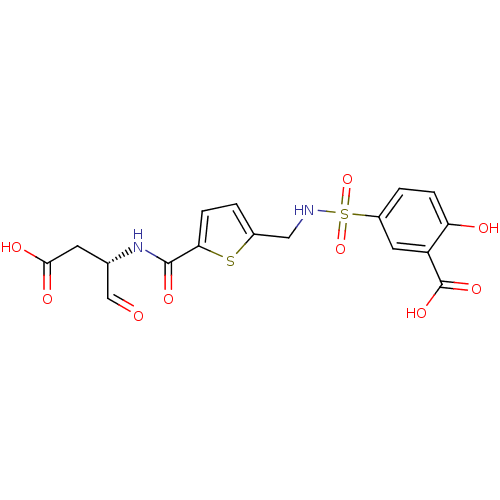
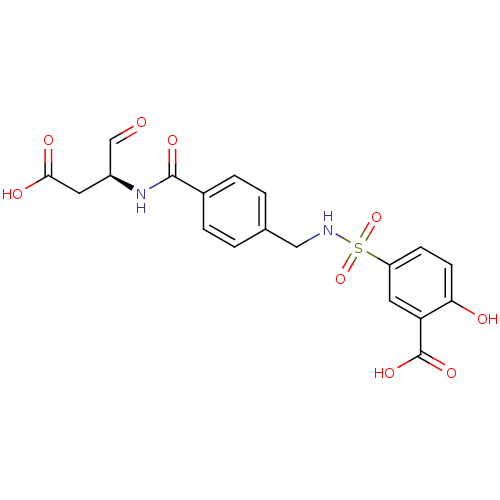
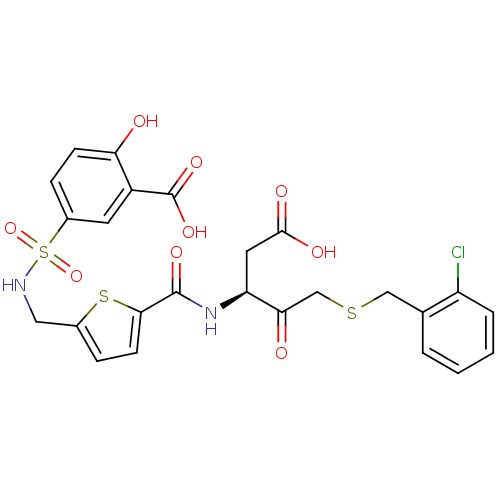
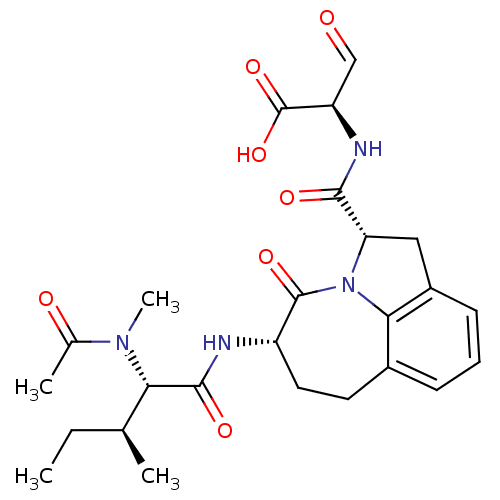
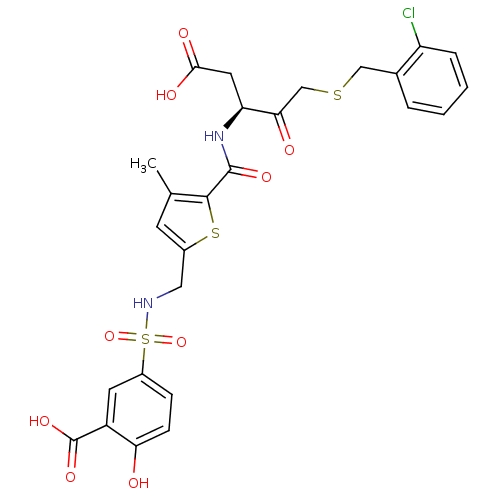
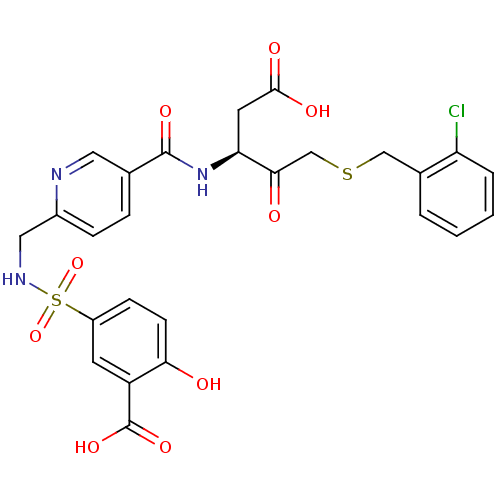
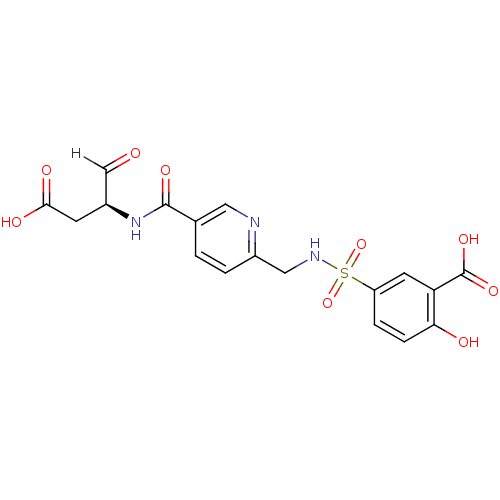
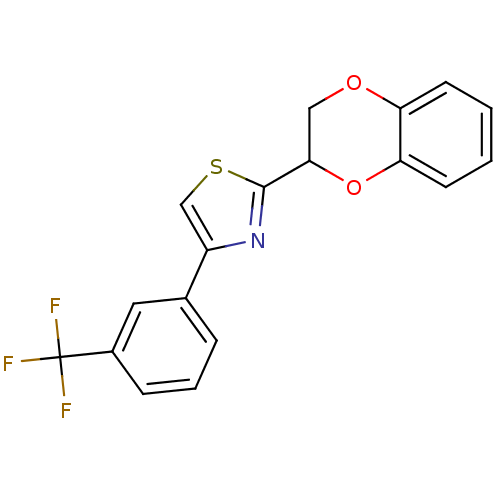
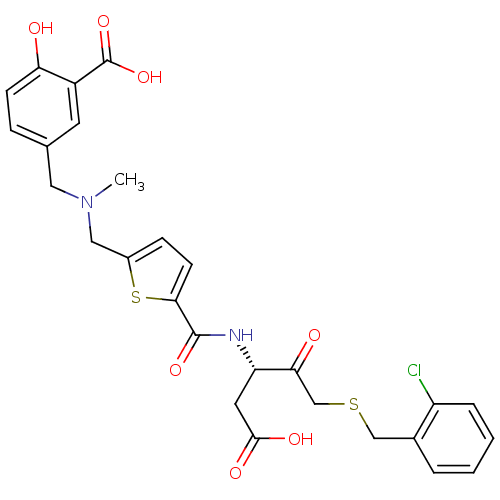
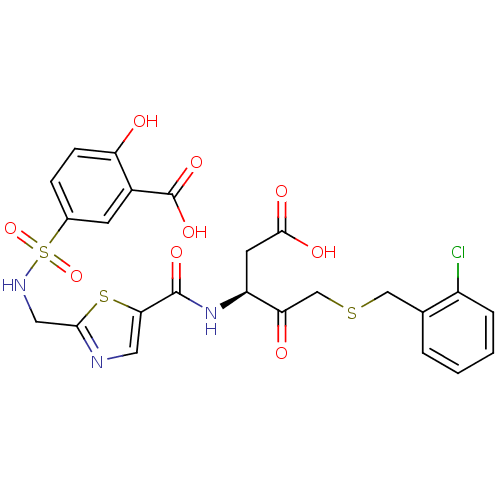
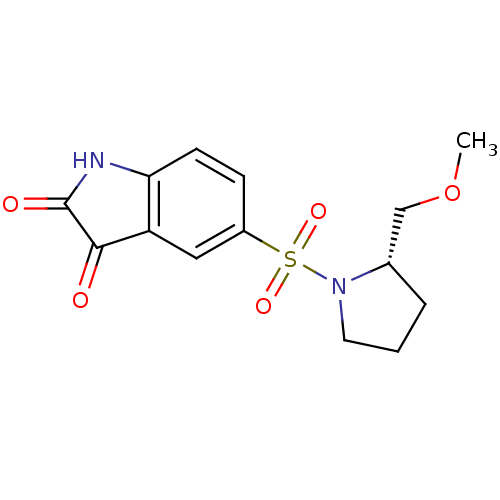
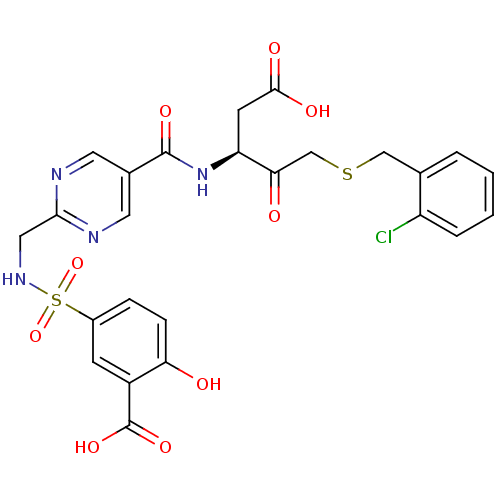
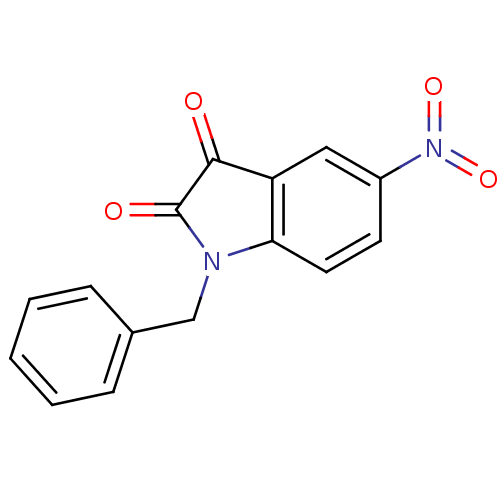
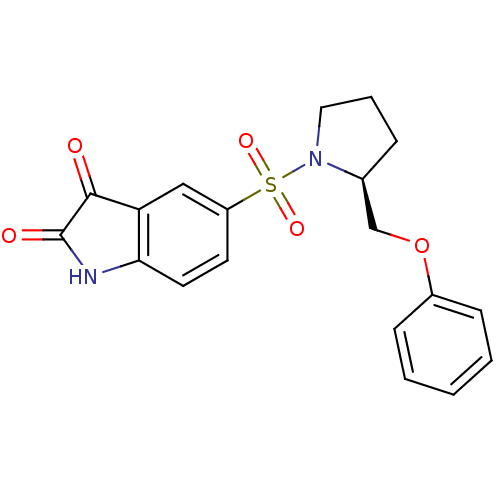
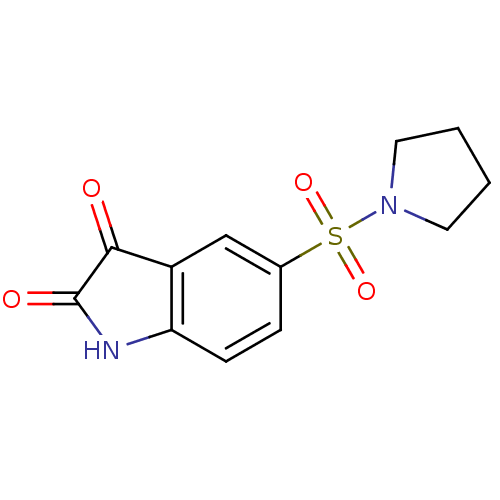
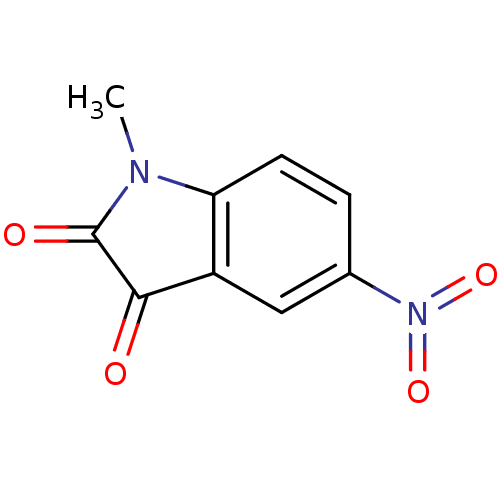
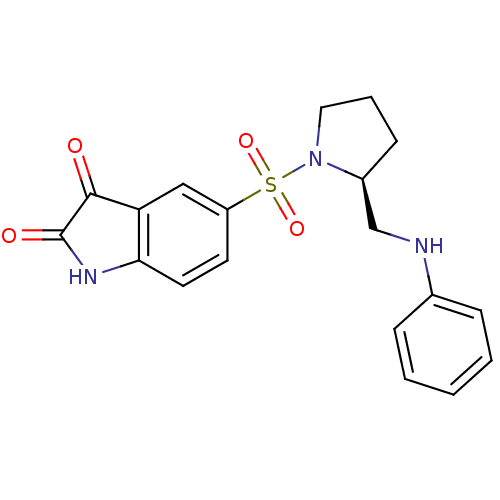
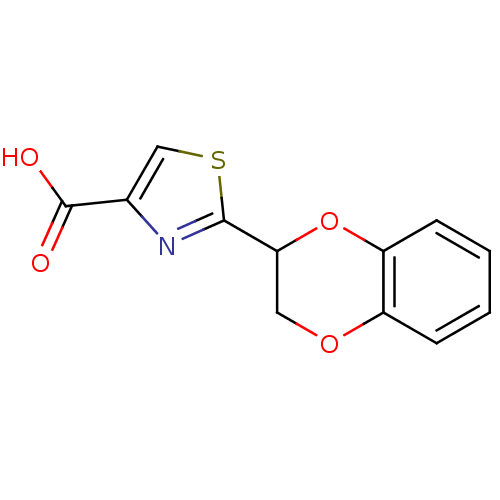
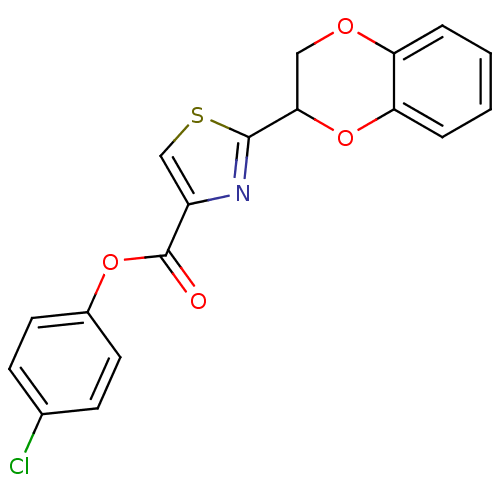
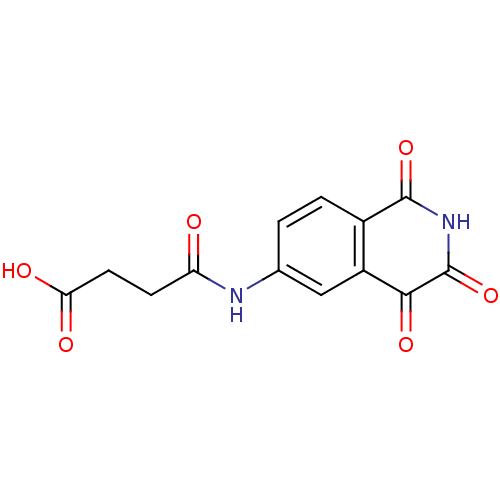
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataEC50: >5.00E+3nMAssay Description:Inhibition of human recombinant caspase 8 assessed as accumulation of 7-amino-4-methylcoumarin substrateMore data for this Ligand-Target Pair
Affinity DataKi: 50nM ΔG°: -41.7kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ...More data for this Ligand-Target Pair
Affinity DataKi: 150nM ΔG°: -38.9kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ...More data for this Ligand-Target Pair
Affinity DataKi: 330nM ΔG°: -37.0kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ...More data for this Ligand-Target Pair
Affinity DataKi: 350nMAssay Description:Compound was tested for its inhibition against Caspase-8More data for this Ligand-Target Pair
Affinity DataKi: 540nMAssay Description:Inhibition of human caspase 8More data for this Ligand-Target Pair
Affinity DataKi: 710nM ΔG°: -35.1kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ...More data for this Ligand-Target Pair
Affinity DataKi: 810nM ΔG°: -34.8kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ...More data for this Ligand-Target Pair
Affinity DataKi: 880nM ΔG°: -34.6kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ...More data for this Ligand-Target Pair
Affinity DataKi: 2.70E+3nMAssay Description:Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a...More data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nM ΔG°: -30.8kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ...More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+3nMAssay Description:The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with...More data for this Ligand-Target Pair
Affinity DataKi: 1.90E+4nM ΔG°: -26.9kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ...More data for this Ligand-Target Pair
Affinity DataKi: >2.50E+4nMAssay Description:The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with...More data for this Ligand-Target Pair
Affinity DataKi: 2.80E+4nM ΔG°: -26.0kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ...More data for this Ligand-Target Pair
Affinity DataKi: 3.00E+4nMAssay Description:Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a...More data for this Ligand-Target Pair
Affinity DataKi: 3.10E+4nMAssay Description:The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with...More data for this Ligand-Target Pair
Affinity DataKi: 4.90E+4nMAssay Description:The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with...More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMAssay Description:The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with...More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMAssay Description:The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with...More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMAssay Description:The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with...More data for this Ligand-Target Pair
Affinity DataKi: 8.00E+4nMAssay Description:The substrate peptides terminating in AMC/AFC are processed by caspases with or without inhibitors, and the accumulation of AMC/AFC was assessed with...More data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+4nMAssay Description:Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a...More data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+4nMAssay Description:Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a...More data for this Ligand-Target Pair
Affinity DataIC50: 6.20E+4nMAssay Description:Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a...More data for this Ligand-Target Pair
Affinity DataIC50: 7.50E+4nMAssay Description:Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+5nMAssay Description:Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+5nMAssay Description:Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+5nMAssay Description:Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+5nMAssay Description:Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+5nMAssay Description:Fluorescent assays were carried out in black 96-well plates. Caspase activity was monitored using a Labsystem Fluoroskan II spectrofluorometer with a...More data for this Ligand-Target Pair
Affinity DataIC50: 1.91E+3nMAssay Description:The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I...More data for this Ligand-Target Pair
Affinity DataIC50: 425nMAssay Description:The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I...More data for this Ligand-Target Pair
Affinity DataIC50: 575nMAssay Description:The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I...More data for this Ligand-Target Pair
Affinity DataIC50: 1.12E+3nMAssay Description:The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I...More data for this Ligand-Target Pair
Affinity DataIC50: 288nMAssay Description:The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I...More data for this Ligand-Target Pair
Affinity DataIC50: 835nMAssay Description:The rate of chromogenic substrate hydrolysis was monitored by the change of absorbance at 405 nm for 3 min. Compounds were tested in duplicate. The I...More data for this Ligand-Target Pair