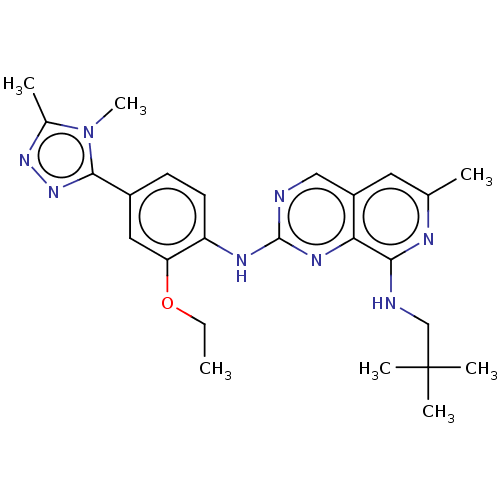
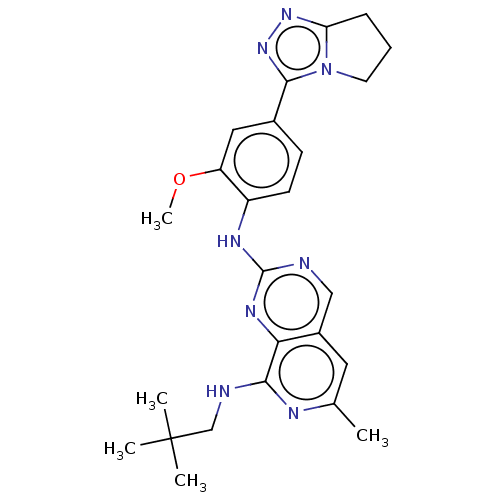
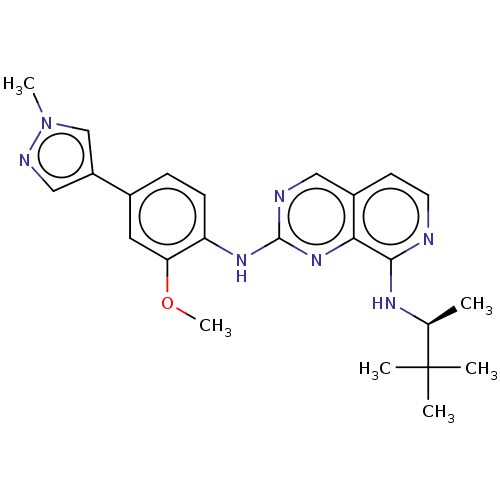
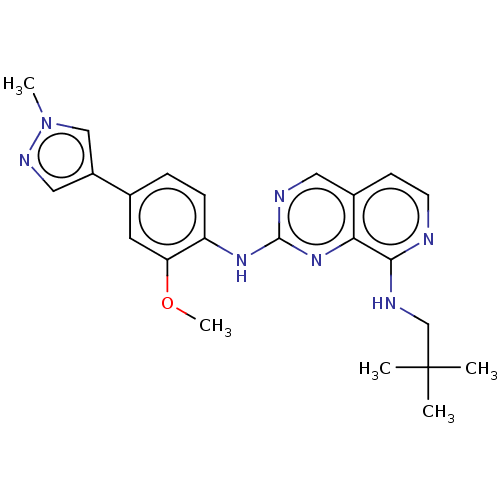
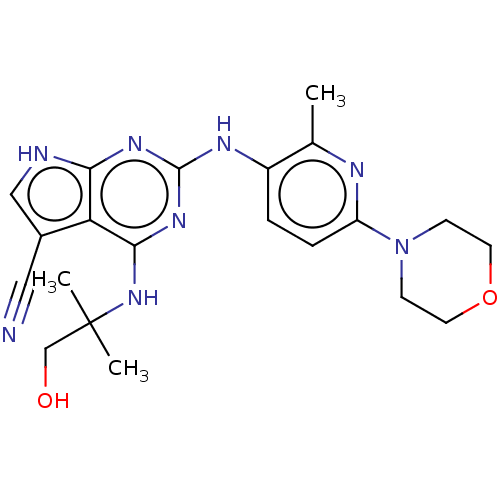
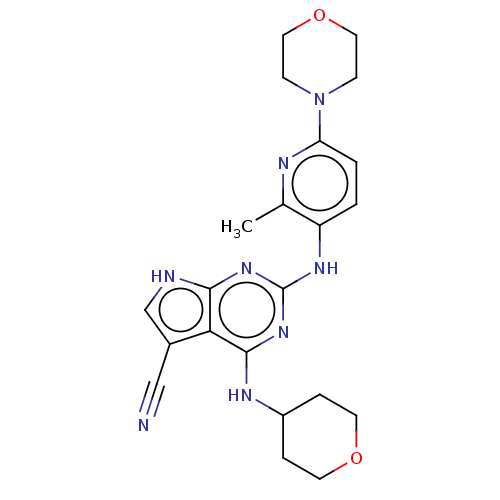
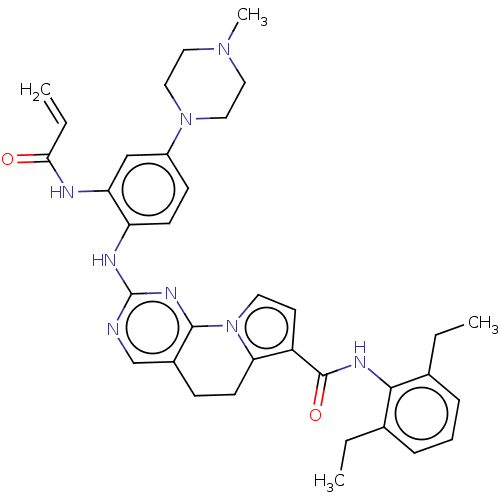
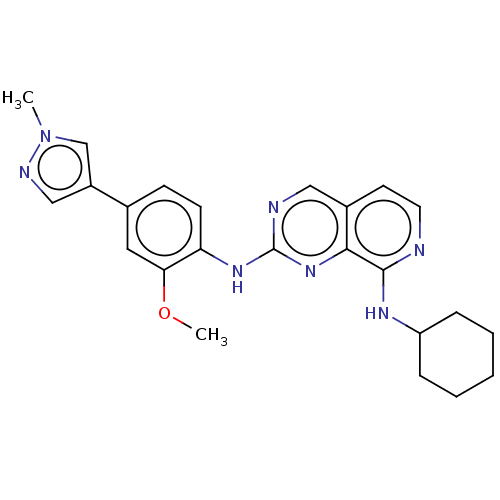
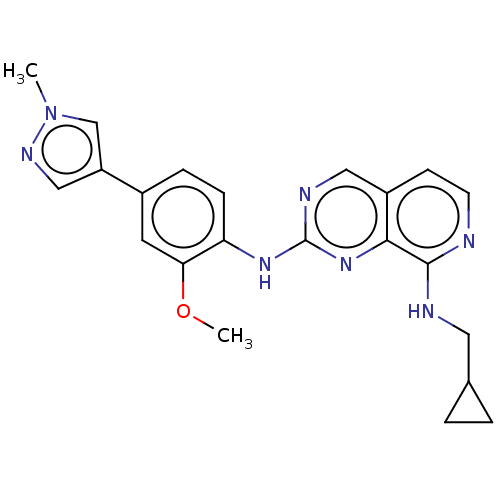
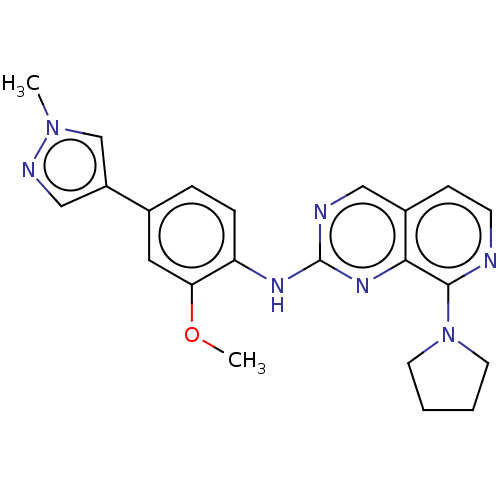
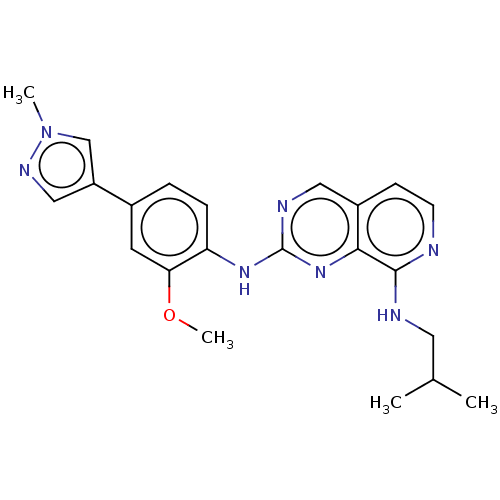
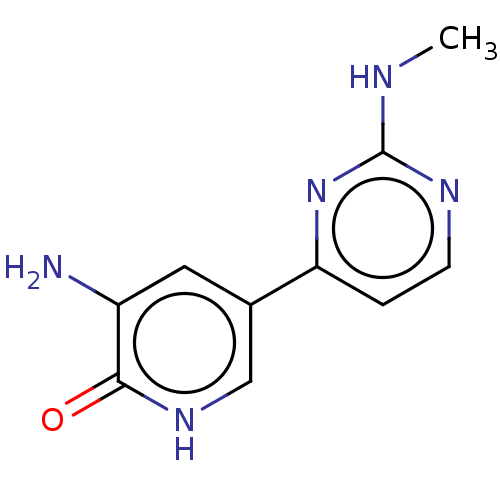
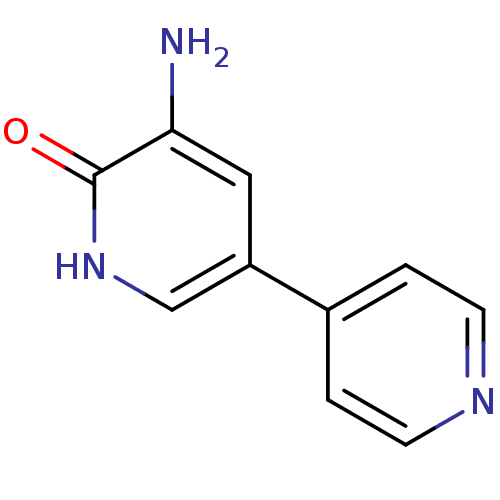
Affinity DataEC50: 14nMAssay Description:Inhibition of TTK (unknown origin) transfected in human HCT116 cells after 4 hrs by near infrared imager analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 420nMAssay Description:Inhibition of TTK in human HCT116 cells assessed as phosphorylation of histone H3 at Ser10 residue after 4 hrs by immunoassayMore data for this Ligand-Target Pair
Affinity DataEC50: 520nMAssay Description:Inhibition of TTK in human HCT116 cells assessed as phosphorylation of histone H3 at Ser10 residue after 4 hrs by immunoassayMore data for this Ligand-Target Pair
Affinity DataKi: 0.0470nMAssay Description:Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60...More data for this Ligand-Target Pair
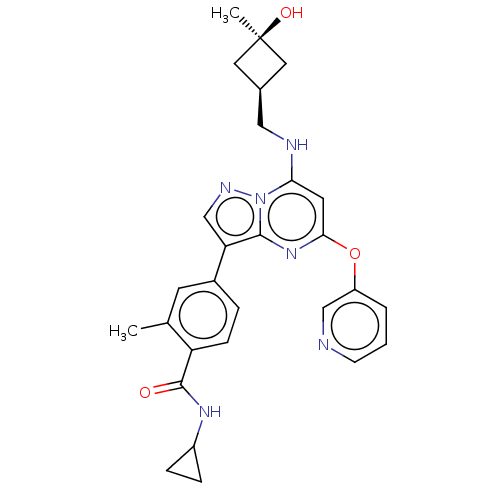
Ligand Info
Affinity DataKi: 0.0840nMAssay Description:Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60...More data for this Ligand-Target Pair
Affinity DataKi: 0.0880nMAssay Description:Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60...More data for this Ligand-Target Pair
Affinity DataKi: 0.0940nMAssay Description:Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60...More data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Inhibition of full length recombinant N-terminal GST-tagged and sumo-tagged human TTK (1 to 275 residues) expressed in Escherichia coli pre-incubated...More data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Inhibition of full length recombinant N-terminal GST-tagged and sumo-tagged human TTK (1 to 275 residues) expressed in Escherichia coli pre-incubated...More data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60...More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 0.110nMAssay Description:Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60...More data for this Ligand-Target Pair
Affinity DataKi: 0.120nMAssay Description:Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60...More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 0.120nMAssay Description:Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60...More data for this Ligand-Target Pair
Affinity DataKi: 0.130nMAssay Description:Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60...More data for this Ligand-Target Pair
Affinity DataKi: 0.190nMAssay Description:Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60...More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 0.380nMAssay Description:Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60...More data for this Ligand-Target Pair
Affinity DataKi: 0.440nMAssay Description:Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60...More data for this Ligand-Target Pair
Affinity DataKi: <0.5nMAssay Description:Inhibition of MPS1 (510 to 857 residues) catalytic domain (unknown origin) expressed in Escherichia coli after 15 mins by mass-spectrometry analysisMore data for this Ligand-Target Pair
Affinity DataKi: <0.5nMAssay Description:Inhibition of MPS1 (510 to 857 residues) catalytic domain (unknown origin) expressed in Escherichia coli after 15 mins by mass-spectrometry analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Competitive inhibition of amino terminal GST-fused full length human TTK using His6-SUMO-TTK-N as substrate by Lineweaver-Burk plot analysis in prese...More data for this Ligand-Target Pair
Affinity DataKi: 0.770nMAssay Description:Competitive inhibition of TTK (unknown origin) by double reciprocal plot analysis in presence of ATPMore data for this Ligand-Target Pair
Affinity DataKi: 0.780nMAssay Description:Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60...More data for this Ligand-Target Pair
Affinity DataKi: 0.820nMAssay Description:Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60...More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 0.830nMAssay Description:Inhibition of recombinant human full length N-terminal GST-fused MPS1 (1 to 857 residues) using histone H3 as substrate by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ...More data for this Ligand-Target Pair
Affinity DataKi: 2.80nMAssay Description:Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ...More data for this Ligand-Target Pair
Affinity DataKi: 3.30nMAssay Description:Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ...More data for this Ligand-Target Pair
Affinity DataKi: 3.70nMAssay Description:Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60...More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 4.20nMAssay Description:Inhibition of human full length MPS1 expressed in recombinant baculovirus infected Sf9 insect cells using 5FAM-DHTGFLTEYVATRCONH2 as substrate after ...More data for this Ligand-Target Pair
Affinity DataKi: 14.2nMAssay Description:Covalent inhibition of human full length N-terminal GST-fused MPS1 (1 to 857 residues) assessed as inhibition constant by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Inhibition of full length human N-terminal 6xHis-tagged MPS1 expressed in baculovirus expression system using 5FAM-H236 peptide as substrate after 60...More data for this Ligand-Target Pair
Affinity DataKi: 150nMAssay Description:Inhibition of TTK (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2.90E+3nMAssay Description:Inhibition of full length human Mps1 using fluorescence-labeled H236 peptide as substrate after 60 to 90 mins in presence of ATP by fluorescence assa...More data for this Ligand-Target Pair
Affinity DataKi: 3.20E+3nMAssay Description:Inhibition of full length human Mps1 using fluorescence-labeled H236 peptide as substrate after 60 to 90 mins in presence of ATP by fluorescence assa...More data for this Ligand-Target Pair
Affinity DataKi: 4.80E+3nMAssay Description:Inhibition of full length human Mps1 using fluorescence-labeled H236 peptide as substrate after 60 to 90 mins in presence of ATP by fluorescence assa...More data for this Ligand-Target Pair
Affinity DataKi: 5.40E+3nMAssay Description:Inhibition of full length human Mps1 using fluorescence-labeled H236 peptide as substrate after 60 to 90 mins in presence of ATP by fluorescence assa...More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+4nMAssay Description:Inhibition of full length human Mps1 using fluorescence-labeled H236 peptide as substrate after 60 to 90 mins in presence of ATP by fluorescence assa...More data for this Ligand-Target Pair
Affinity DataKi: 2.48E+4nMAssay Description:Inhibition of full length human Mps1 using fluorescence-labeled H236 peptide as substrate after 60 to 90 mins in presence of ATP by fluorescence assa...More data for this Ligand-Target Pair
Affinity DataKi: 2.56E+4nMAssay Description:Inhibition of full length human Mps1 using fluorescence-labeled H236 peptide as substrate after 60 to 90 mins in presence of ATP by fluorescence assa...More data for this Ligand-Target Pair
Affinity DataKi: 3.01E+4nMAssay Description:Inhibition of full length human Mps1 using fluorescence-labeled H236 peptide as substrate after 60 to 90 mins in presence of ATP by fluorescence assa...More data for this Ligand-Target Pair
Affinity DataKi: 3.02E+4nMAssay Description:Inhibition of full length human Mps1 using fluorescence-labeled H236 peptide as substrate after 60 to 90 mins in presence of ATP by fluorescence assa...More data for this Ligand-Target Pair
Affinity DataKi: 8.08E+4nMAssay Description:Inhibition of full length human Mps1 using fluorescence-labeled H236 peptide as substrate after 60 to 90 mins in presence of ATP by fluorescence assa...More data for this Ligand-Target Pair
Affinity DataKi: 9.12E+4nMAssay Description:Inhibition of full length human Mps1 using fluorescence-labeled H236 peptide as substrate after 60 to 90 mins in presence of ATP by fluorescence assa...More data for this Ligand-Target Pair
Affinity DataKi: 9.47E+4nMAssay Description:Inhibition of full length human Mps1 using fluorescence-labeled H236 peptide as substrate after 60 to 90 mins in presence of ATP by fluorescence assa...More data for this Ligand-Target Pair
Affinity DataKi: 9.81E+4nMAssay Description:Inhibition of full length human Mps1 using fluorescence-labeled H236 peptide as substrate after 60 to 90 mins in presence of ATP by fluorescence assa...More data for this Ligand-Target Pair

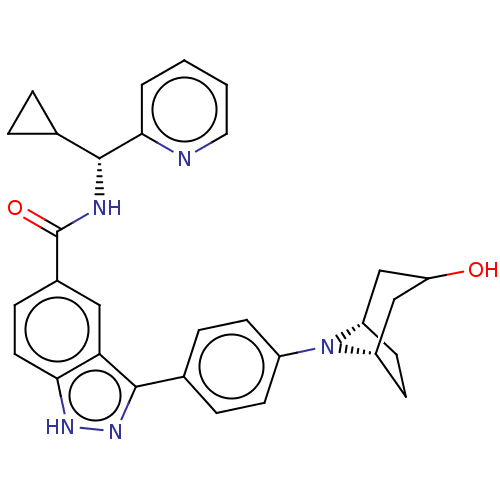

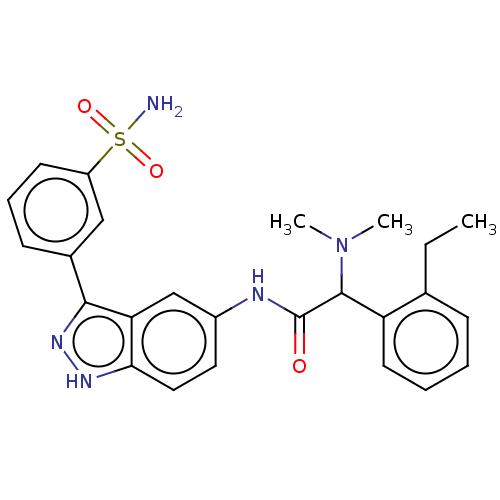


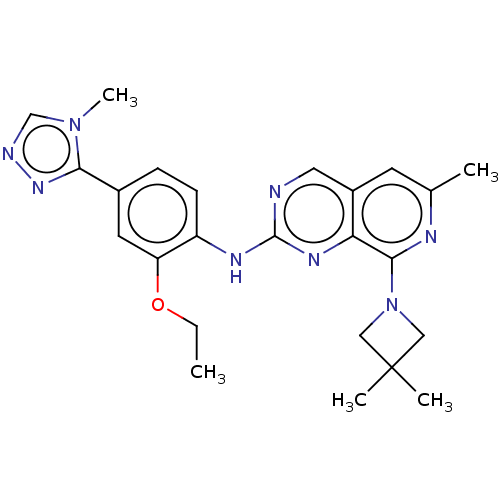



 3D Structure (crystal)
3D Structure (crystal)