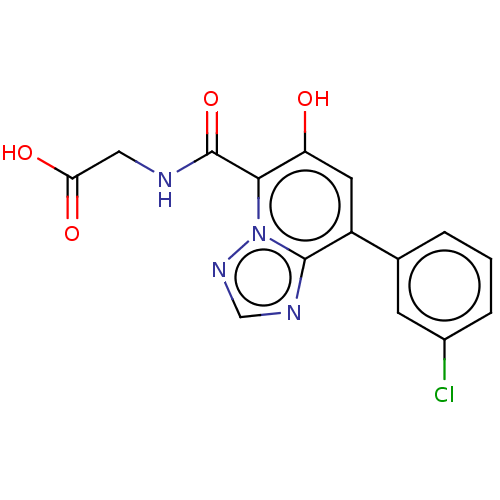
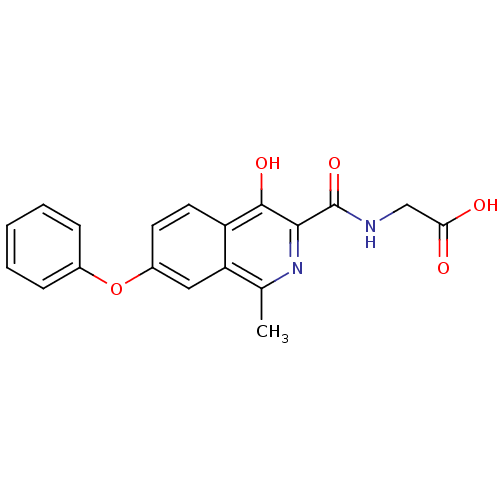
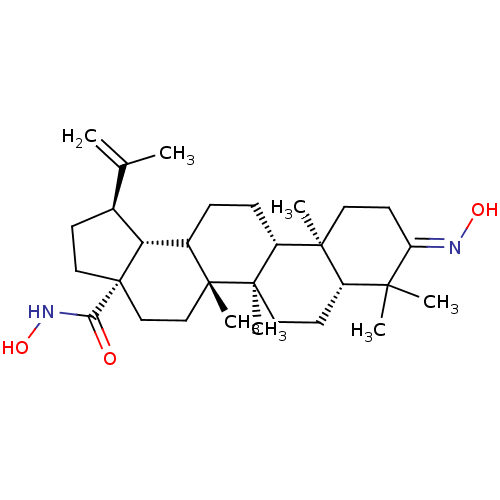
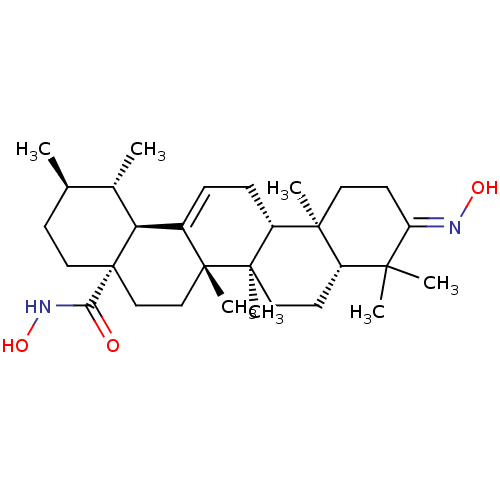
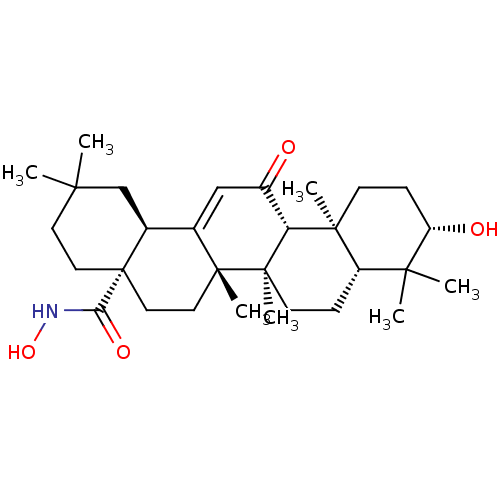
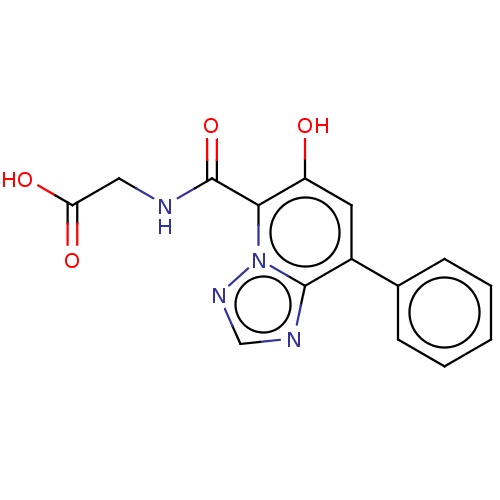
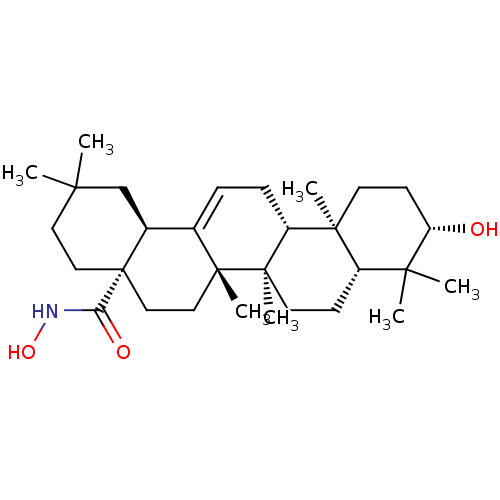
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 10nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO production measured after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 100nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO production measured after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 190nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO production measured after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 1.25E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO production measured after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 2.40E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 2.40E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 2.60E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 2.60E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 3.20E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 3.20E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 3.80E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 3.80E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 4.20E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO release after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 4.46E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO production measured after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 4.50E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO release after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 4.70E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO release after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 4.80E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 4.80E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 4.91E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO production measured after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 5.00E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 5.00E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 5.54E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO production measured after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 5.70E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO release after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 5.80E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 5.80E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO release after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 5.80E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO release after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 5.80E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO release after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 5.80E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 6.11E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO production measured after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 6.80E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 6.80E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 7.10E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 7.10E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 7.50E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 7.50E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 7.70E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 7.70E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 7.97E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO production measured after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 8.90E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 8.90E+3nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 9.27E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO production measured after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 9.30E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO release after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 1.14E+4nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 1.14E+4nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 1.20E+4nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO release after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 1.30E+4nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO release after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 1.40E+4nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO release after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 1.40E+4nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO release after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1(Homo sapiens (Human))
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Tianjin Institute Of Medical & Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataEC50: 1.50E+4nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as increase in EPO release after 24 hrs by ELISAMore data for this Ligand-Target Pair
TargetEgl nine homolog 1/Prolyl hydroxylase EGLN2/Prolyl hydroxylase EGLN3/Transmembrane prolyl 4-hydroxylase(Homo sapiens (Human))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 1.64E+4nMAssay Description:Inhibition of PHD (unknown origin) expressed in mouse NIH/3T3 cells harboring HRE-driven luciferase gene assessed as transactivation of HIF1alpha aft...More data for this Ligand-Target Pair