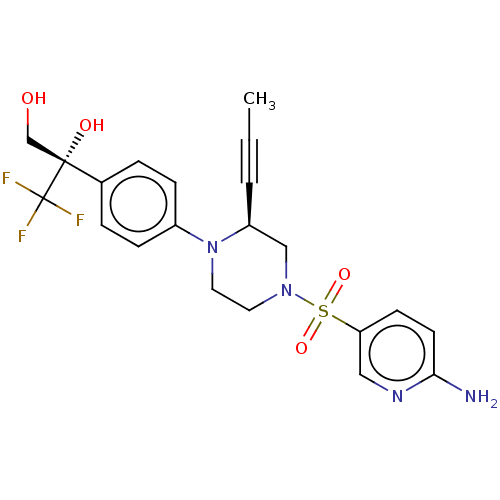
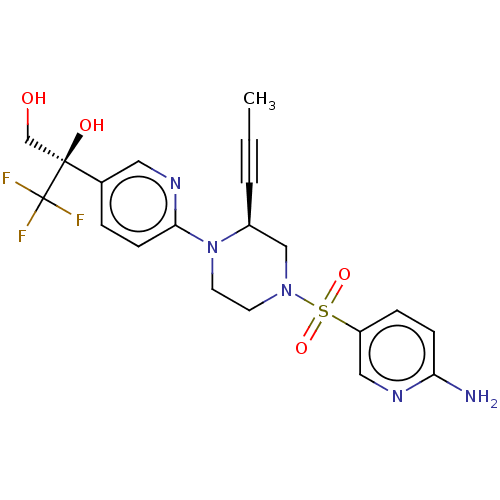
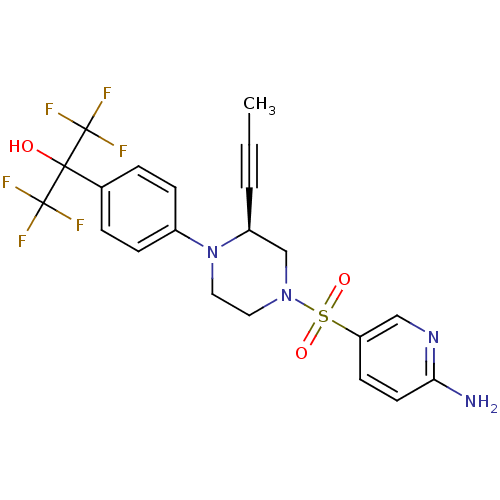
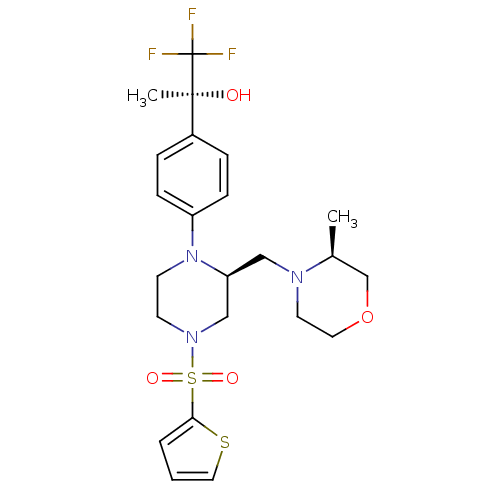
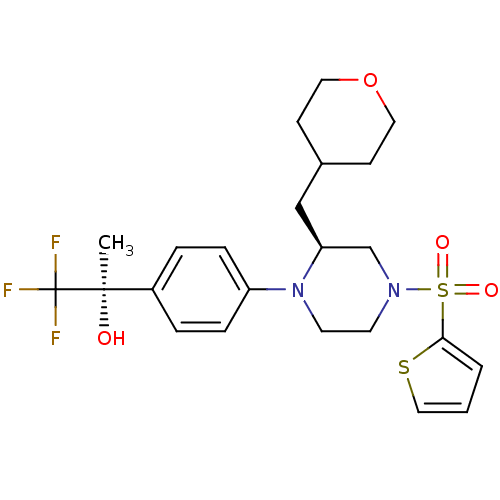
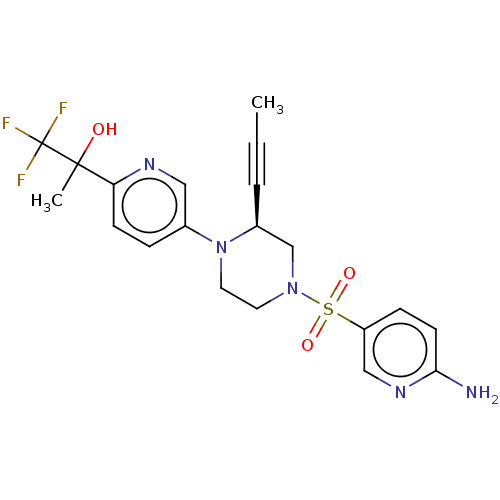
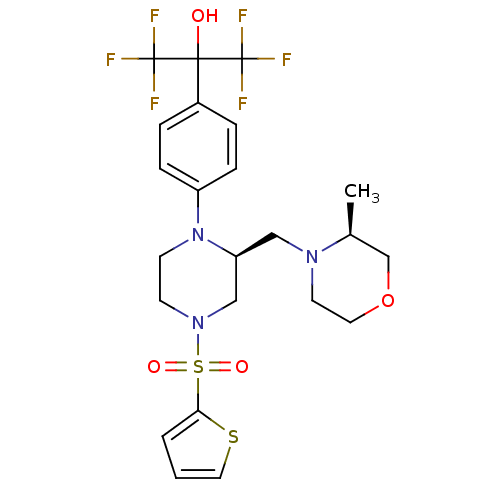
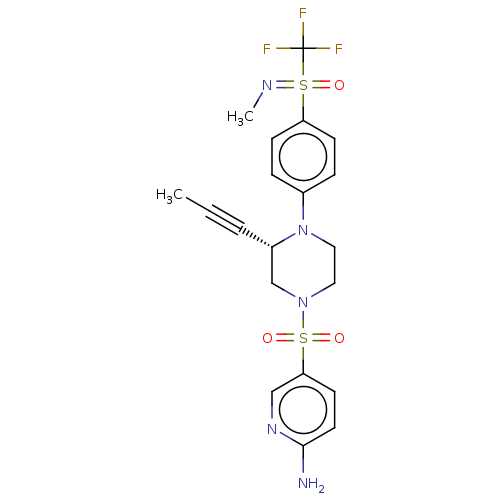
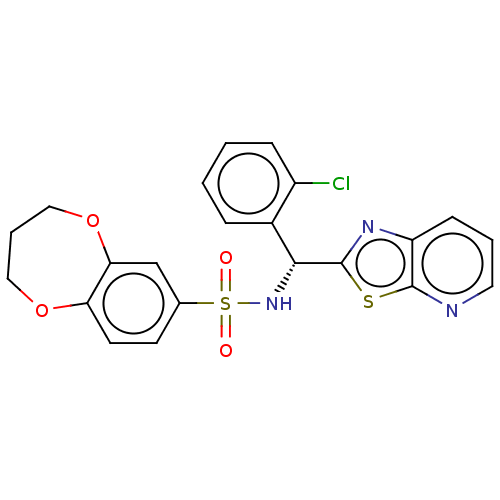
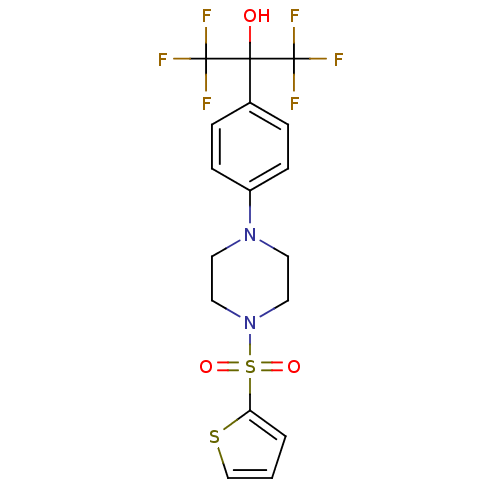
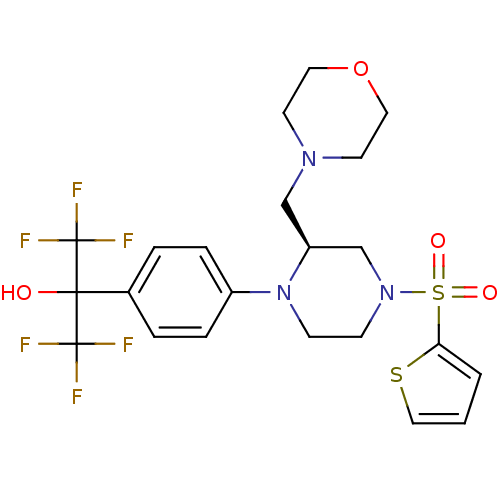
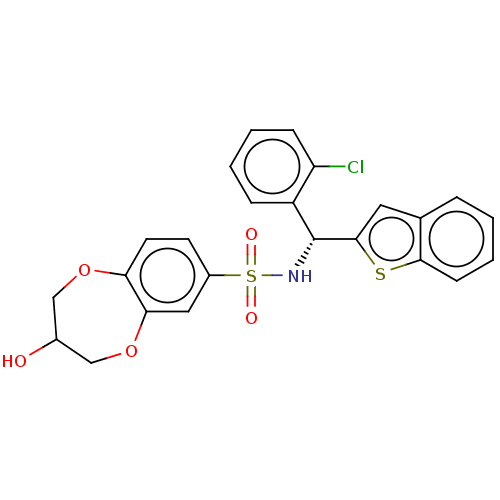
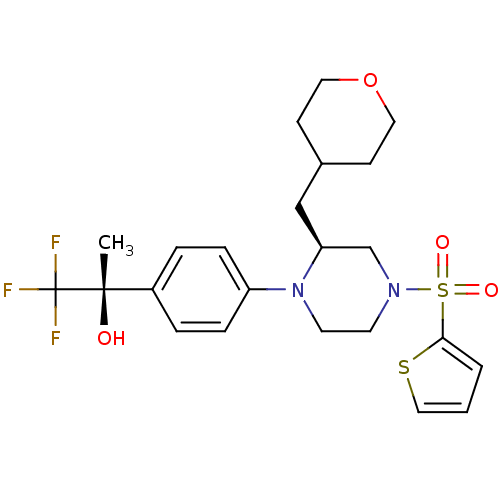
Affinity DataEC50: 37nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 66nMAssay Description:Inhibition of glucokinase-GKRP binding interaction in mouse hepatocytes assessed as induction of glucokinase translocationMore data for this Ligand-Target Pair
Affinity DataEC50: 82nMAssay Description:Inhibition of GKRP-GK interaction in rat primary hepatocytes assessed as translocation of GK from nucleus to cytoplasm preincubated for 20 mins follo...More data for this Ligand-Target Pair
Affinity DataEC50: 89nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 97nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 98nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 120nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 130nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 137nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 145nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 155nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 160nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 178nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 202nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 202nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 208nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 219nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 220nMAssay Description:Inhibition of GKRP-GK interaction in rat primary hepatocytes assessed as translocation of GK from nucleus to cytoplasm preincubated for 20 mins follo...More data for this Ligand-Target Pair
Affinity DataEC50: 254nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 260nMAssay Description:Binding affinity to human GKRP assessed as inhibition of protein interaction with human glucokinase by measuring ATP depletion after 60 mins by lucif...More data for this Ligand-Target Pair
Affinity DataEC50: 271nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 294nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 349nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 381nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 382nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 388nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 410nMAssay Description:Inhibition of glucokinase-GKRP binding interaction in mouse hepatocytes assessed as induction of glucokinase translocationMore data for this Ligand-Target Pair
Affinity DataEC50: 421nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 430nMAssay Description:Inhibition of glucokinase-GKRP binding interaction in mouse hepatocytes assessed as induction of glucokinase translocationMore data for this Ligand-Target Pair
Affinity DataEC50: 480nMAssay Description:Inhibition of glucokinase-GKRP binding interaction in mouse hepatocytes assessed as induction of glucokinase translocationMore data for this Ligand-Target Pair
Affinity DataEC50: 537nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 567nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 704nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 890nMAssay Description:Inhibition of GKRP-GK interaction in rat primary hepatocytes assessed as translocation of GK from nucleus to cytoplasm preincubated for 20 mins follo...More data for this Ligand-Target Pair
Affinity DataEC50: 936nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 1.45E+3nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 1.56E+3nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 1.81E+3nMAssay Description:Inhibition of GKRP-GK interaction in rat primary hepatocytes assessed as translocation of GK from nucleus to cytoplasm preincubated for 20 mins follo...More data for this Ligand-Target Pair
Affinity DataEC50: 1.90E+3nMAssay Description:Inhibition of glucokinase-GKRP binding interaction in mouse hepatocytes assessed as induction of glucokinase translocationMore data for this Ligand-Target Pair
Affinity DataEC50: 2.06E+3nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 2.20E+3nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 2.22E+3nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 2.90E+3nMAssay Description:Inhibition of glucokinase-GKRP binding interaction in mouse hepatocytes assessed as induction of glucokinase translocationMore data for this Ligand-Target Pair
Affinity DataEC50: 3.30E+3nMAssay Description:Inhibition of glucokinase-GKRP binding interaction in mouse hepatocytes assessed as induction of glucokinase translocationMore data for this Ligand-Target Pair
Affinity DataEC50: 3.64E+3nMAssay Description:Inhibition of GKRP-GK interaction in rat primary hepatocytes assessed as translocation of GK from nucleus to cytoplasm preincubated for 20 mins follo...More data for this Ligand-Target Pair
Affinity DataEC50: 3.99E+3nMAssay Description:Inhibition of GKRP-GK interaction in rat primary hepatocytes assessed as translocation of GK from nucleus to cytoplasm preincubated for 20 mins follo...More data for this Ligand-Target Pair
Affinity DataEC50: 4.05E+3nMAssay Description:Inhibition of GKRP-GK interaction in mouse hepatocytes assessed as induction of GK translocation from nucleus to cytoplasm incubated for 20 mins prio...More data for this Ligand-Target Pair
Affinity DataEC50: 4.10E+3nMAssay Description:Inhibition of glucokinase-GKRP binding interaction in mouse hepatocytes assessed as induction of glucokinase translocationMore data for this Ligand-Target Pair
Affinity DataEC50: 4.30E+3nMAssay Description:Inhibition of glucokinase-GKRP binding interaction in mouse hepatocytes assessed as induction of glucokinase translocationMore data for this Ligand-Target Pair
Affinity DataEC50: 4.76E+3nMAssay Description:Inhibition of GKRP-GK interaction in rat primary hepatocytes assessed as translocation of GK from nucleus to cytoplasm preincubated for 20 mins follo...More data for this Ligand-Target Pair

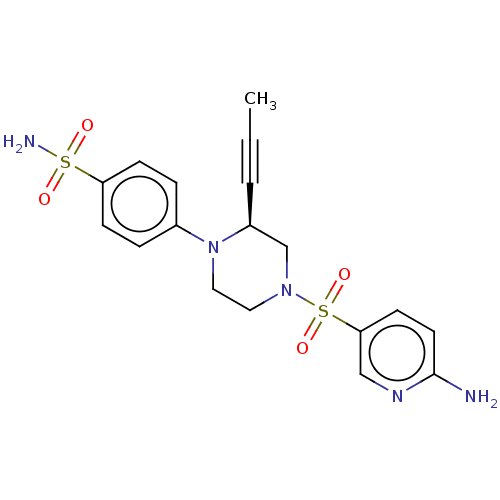

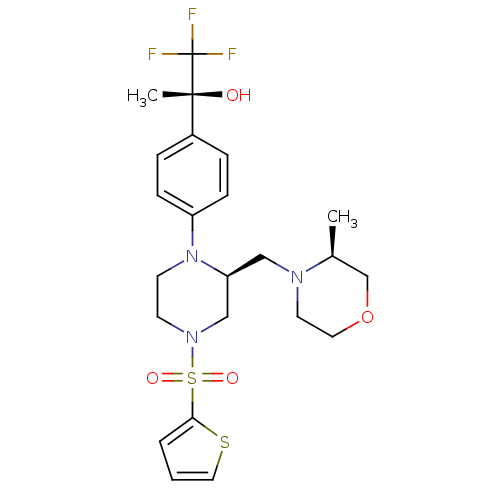
 3D Structure (crystal)
3D Structure (crystal)