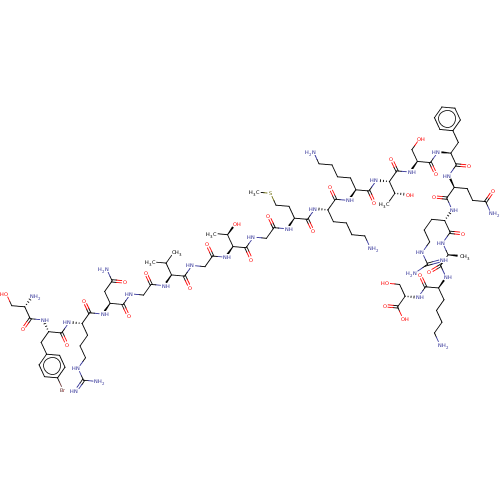
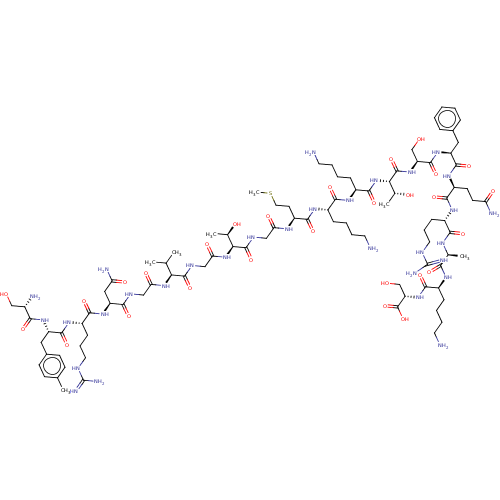
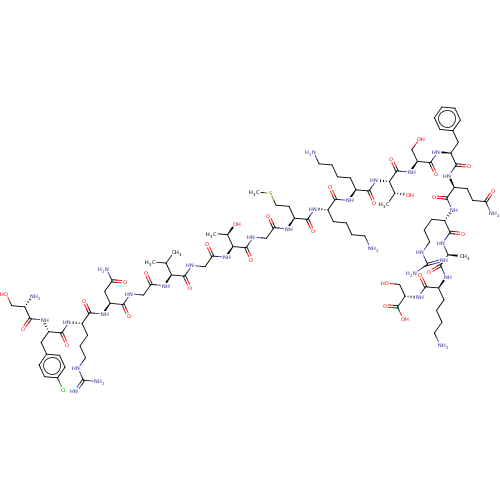
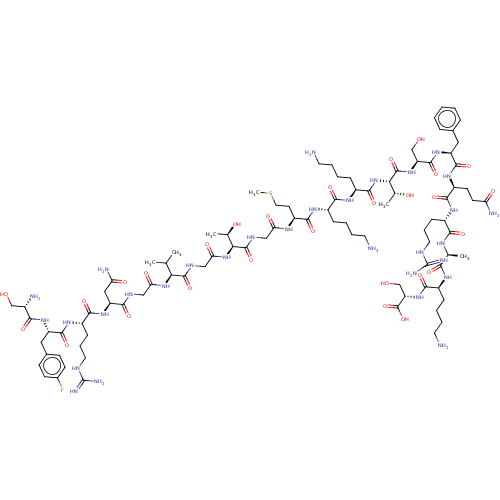
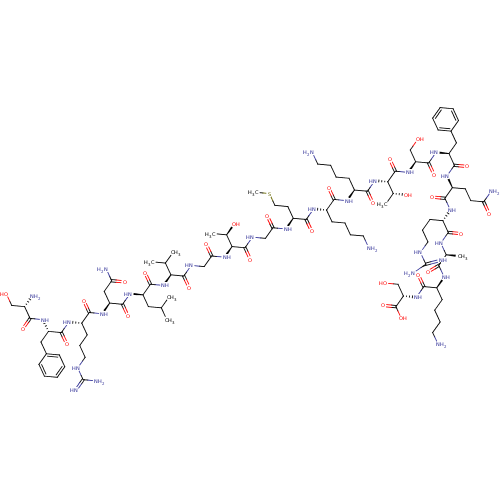
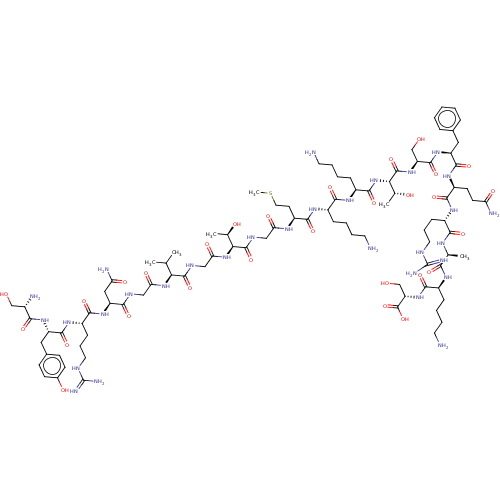
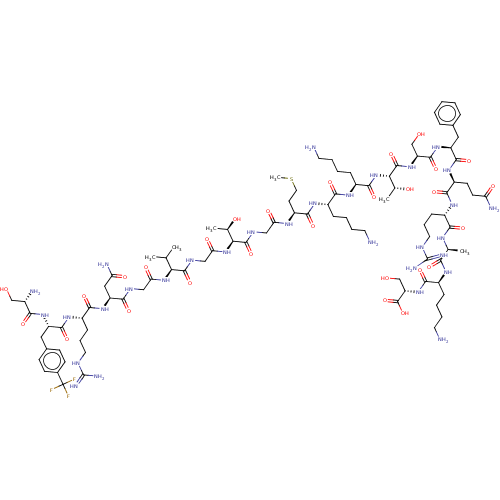
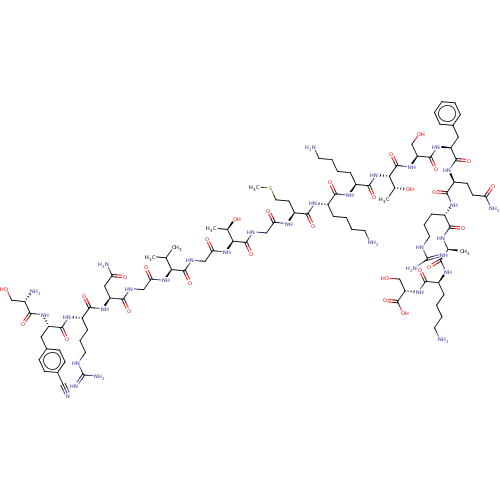
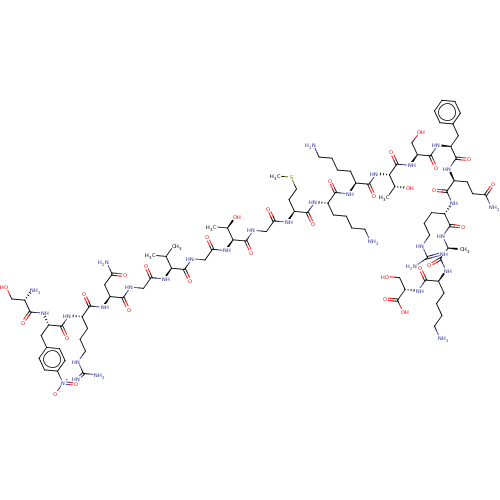
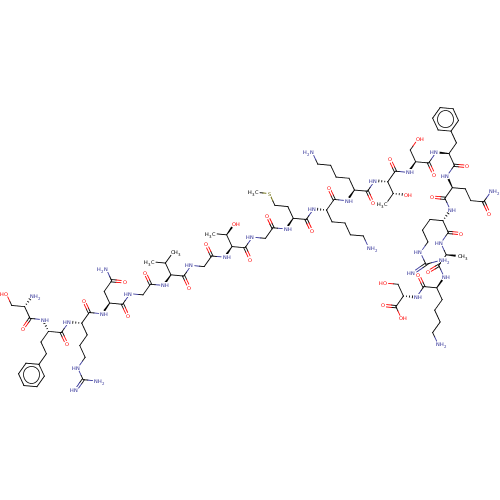
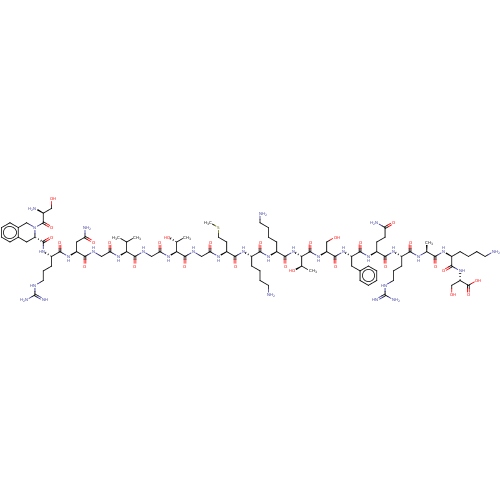
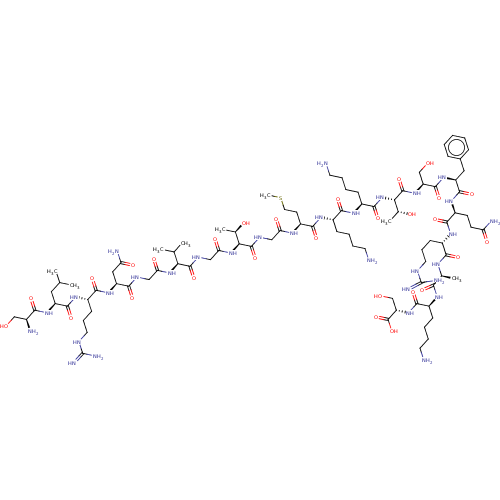
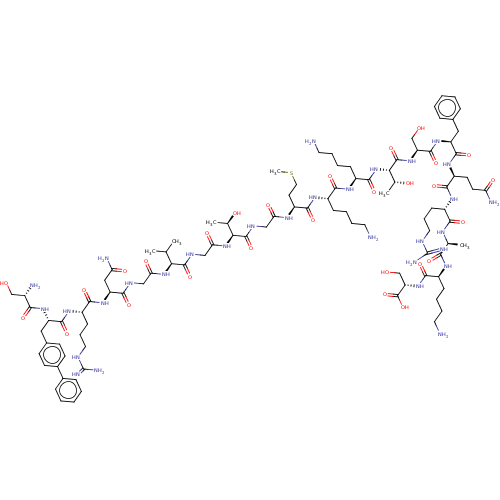
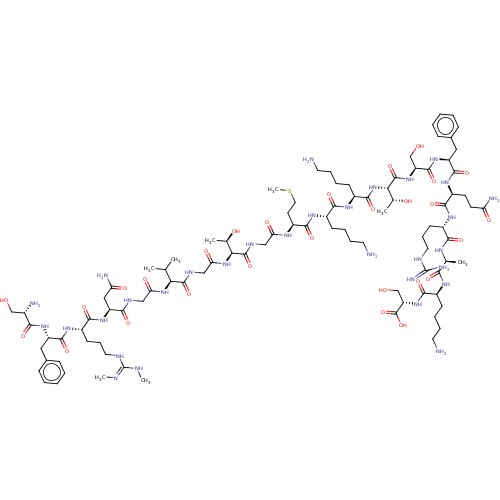
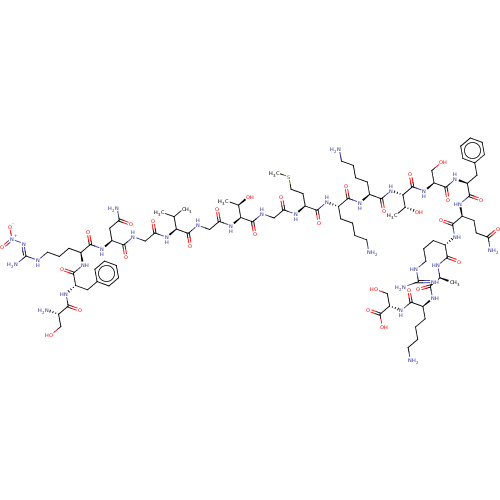
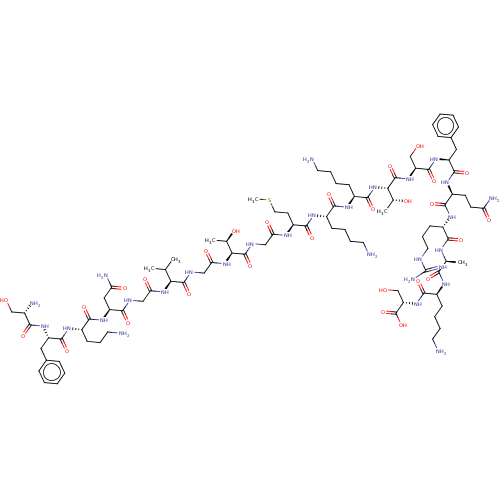
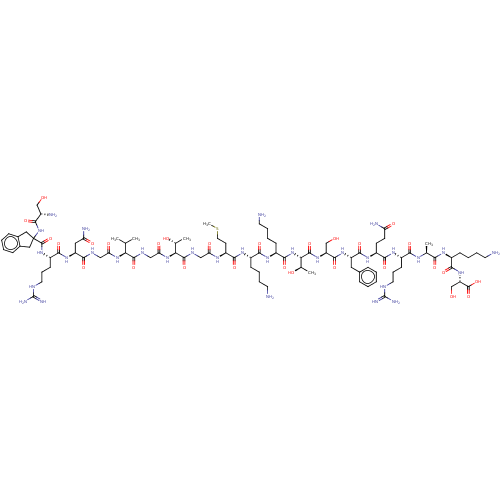
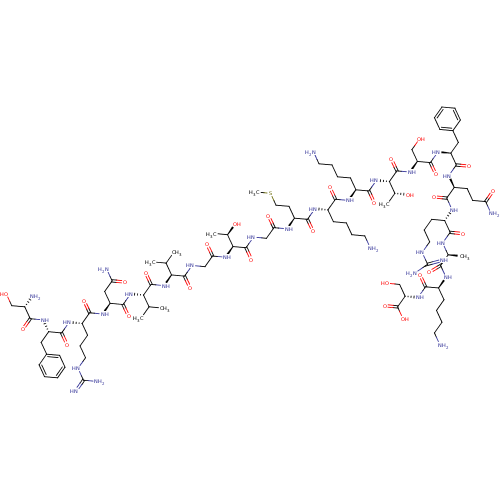
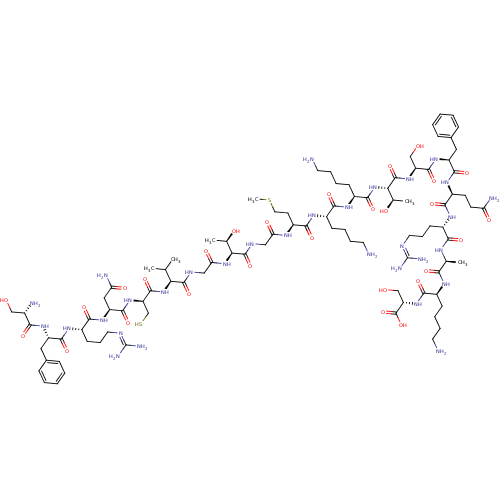
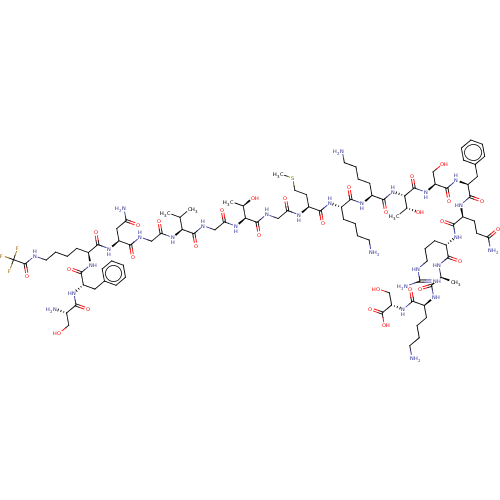
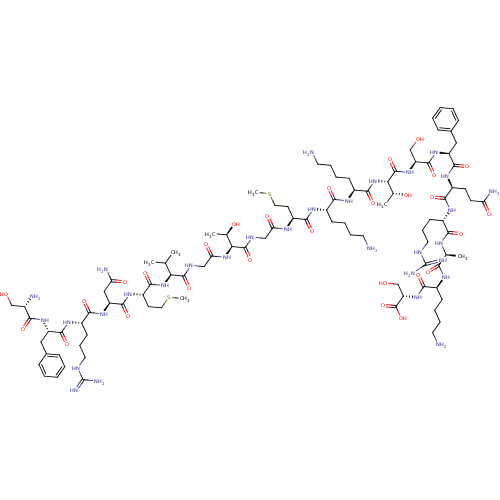
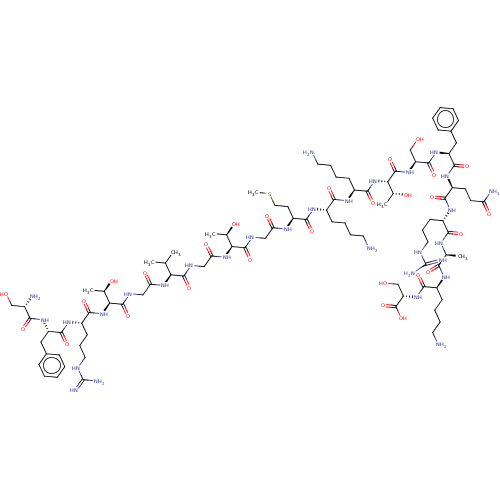
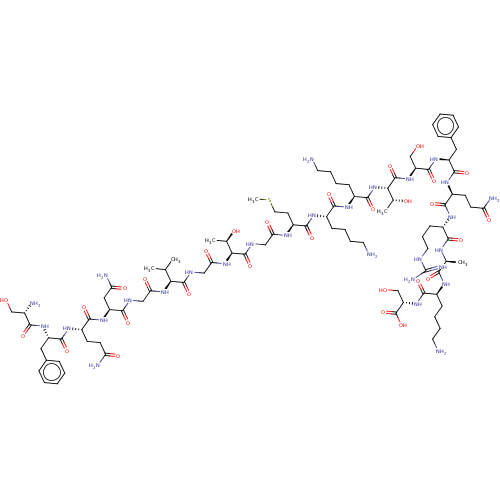
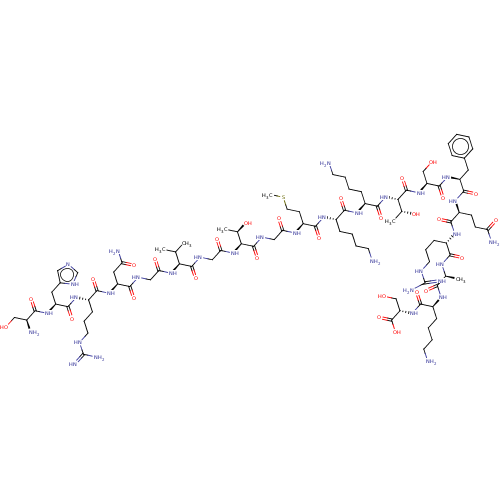
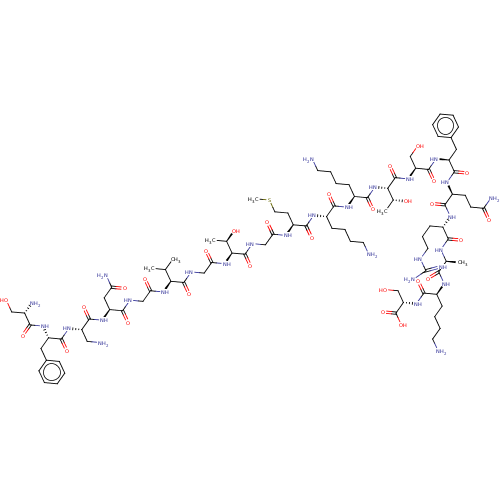
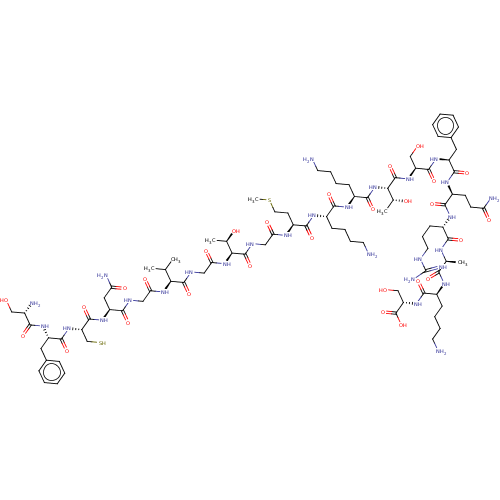
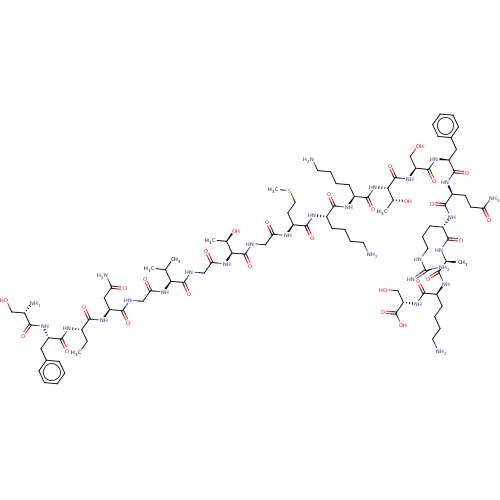
Affinity DataEC50: 0.646nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 0.676nMAssay Description:Agonist activity at human NPSR Ile107 expressed in HEK293 cells assessed as induction of calcium mobilization after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.676nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 0.708nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 0.851nMAssay Description:Agonist activity at human NPSR Asn107 expressed in HEK293 cells assessed as induction of calcium mobilization after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 1.07nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells assessed as induction of calcium mobilization after 30 minsMore data for this Ligand-Target Pair
Affinity DataEC50: 1.10nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 1.10nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 1.30nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 1.80nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 1.90nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 1.90nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 2.20nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 2.20nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 2.24nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells by calcium mobilization assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2.24nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells by calcium mobilization assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2.30nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 2.60nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 3.30nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 3.60nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 4.20nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 4.80nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells by calcium mobilization assayMore data for this Ligand-Target Pair
Affinity DataEC50: 8.10nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 8.30nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 11nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 11nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells assessed as increase in intracellular calcium mobilization by FLIPRMore data for this Ligand-Target Pair
Affinity DataEC50: 12nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells assessed as increase in intracellular calcium mobilization by FLIPRMore data for this Ligand-Target Pair
Affinity DataEC50: 13.8nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells by calcium mobilization assayMore data for this Ligand-Target Pair
Affinity DataEC50: 15nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 18nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells assessed as increase in intracellular calcium mobilization by FLIPRMore data for this Ligand-Target Pair
Affinity DataEC50: 20nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 22nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells assessed as increase in intracellular calcium mobilization by FLIPRMore data for this Ligand-Target Pair
Affinity DataEC50: 22nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells assessed as increase in intracellular calcium mobilization by FLIPRMore data for this Ligand-Target Pair
Affinity DataEC50: 32nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells assessed as increase in intracellular calcium mobilization by FLIPRMore data for this Ligand-Target Pair
Affinity DataEC50: 32nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells assessed as increase in intracellular calcium mobilization by FLIPRMore data for this Ligand-Target Pair
Affinity DataEC50: 34nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 66.1nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells by calcium mobilization assayMore data for this Ligand-Target Pair
Affinity DataEC50: 70.8nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells by calcium mobilization assayMore data for this Ligand-Target Pair
Affinity DataEC50: 85nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells assessed as increase in intracellular calcium mobilization by FLIPRMore data for this Ligand-Target Pair
Affinity DataEC50: 87.1nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells by calcium mobilization assayMore data for this Ligand-Target Pair
Affinity DataEC50: 102nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells assessed as increase in intracellular calcium mobilization by FLIPRMore data for this Ligand-Target Pair
Affinity DataEC50: 129nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells assessed as increase in intracellular calcium mobilization by FLIPRMore data for this Ligand-Target Pair
Affinity DataEC50: 132nMAssay Description:Agonist activity at mouse recombinant NPS receptor expressed in HEK293 cells assessed as intracellular calcium mobilizationMore data for this Ligand-Target Pair
Affinity DataEC50: 135nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells assessed as increase in intracellular calcium mobilization by FLIPRMore data for this Ligand-Target Pair
Affinity DataEC50: 166nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells assessed as increase in intracellular calcium mobilization by FLIPRMore data for this Ligand-Target Pair
Affinity DataEC50: 204nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells assessed as increase in intracellular calcium mobilization by FLIPRMore data for this Ligand-Target Pair
Affinity DataEC50: 229nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells by calcium mobilization assayMore data for this Ligand-Target Pair
Affinity DataEC50: 240nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells assessed as increase in intracellular calcium mobilization by FLIPRMore data for this Ligand-Target Pair
Affinity DataEC50: 372nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells assessed as increase in intracellular calcium mobilization by FLIPRMore data for this Ligand-Target Pair
Affinity DataEC50: 513nMAssay Description:Agonist activity at mouse NPSR expressed in HEK293 cells assessed as increase in intracellular calcium mobilization by FLIPRMore data for this Ligand-Target Pair