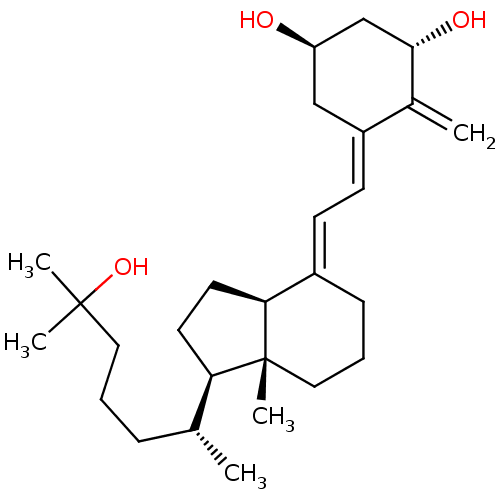
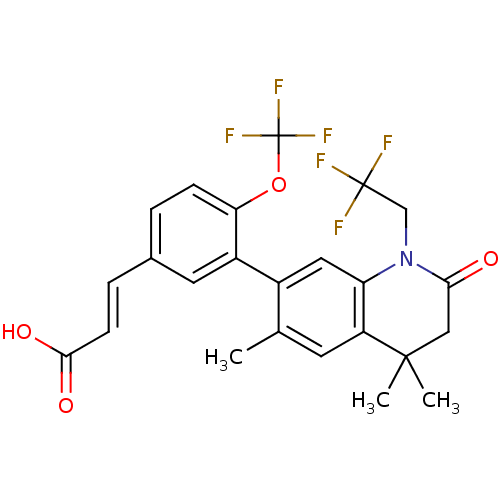
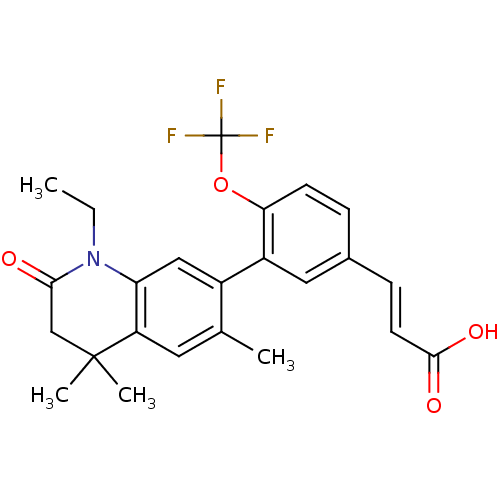
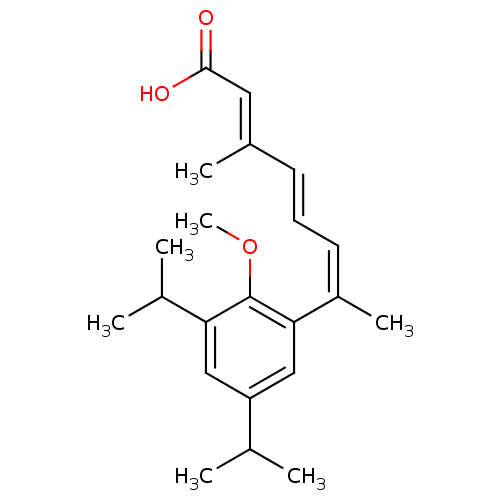
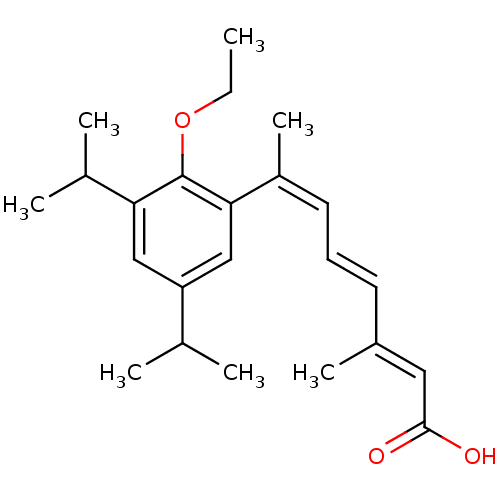
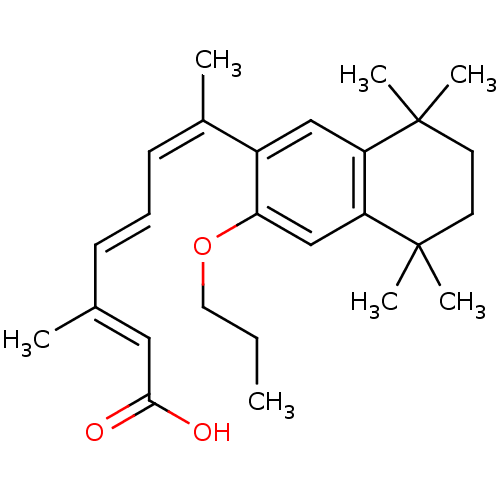
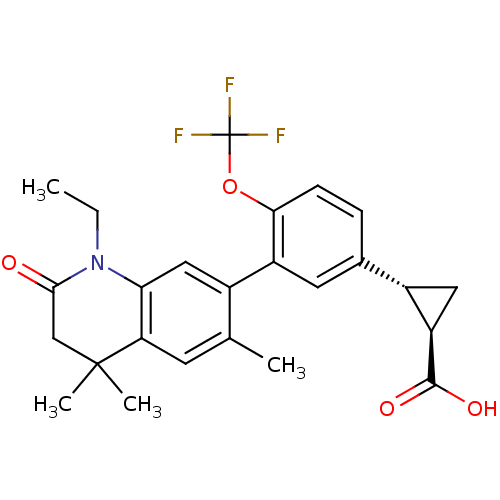
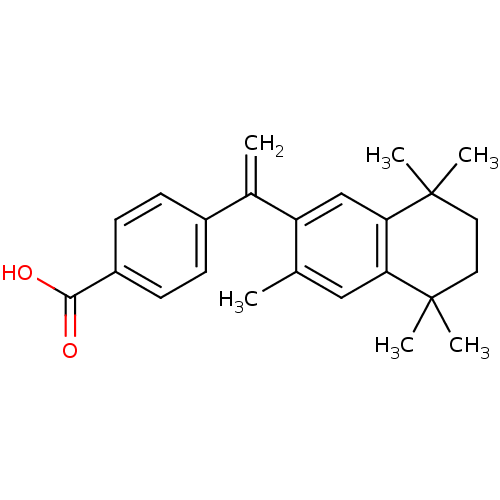
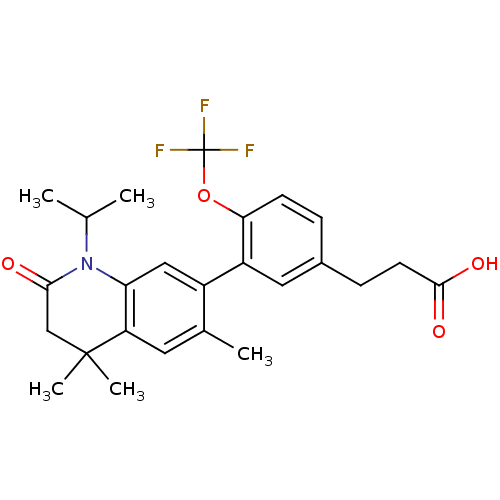
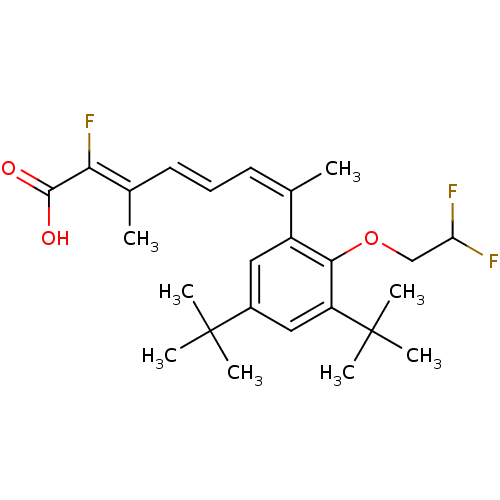
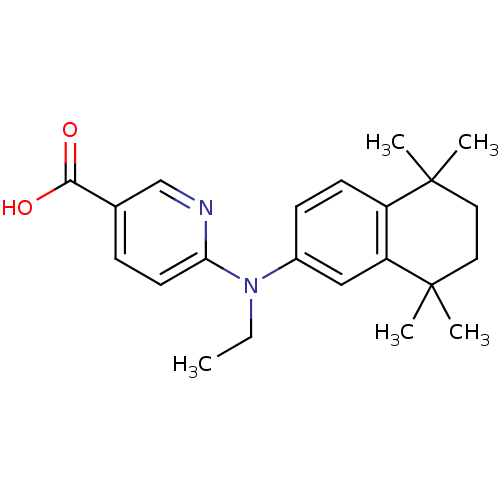
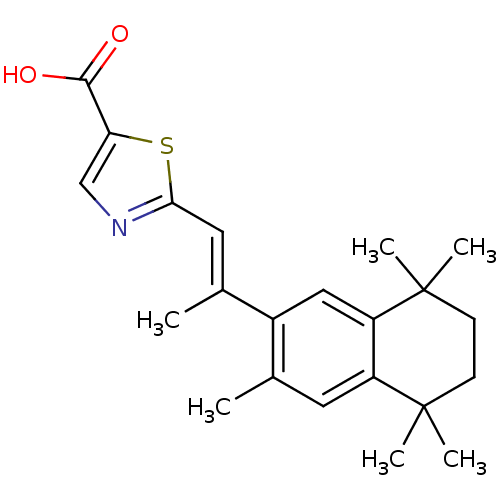
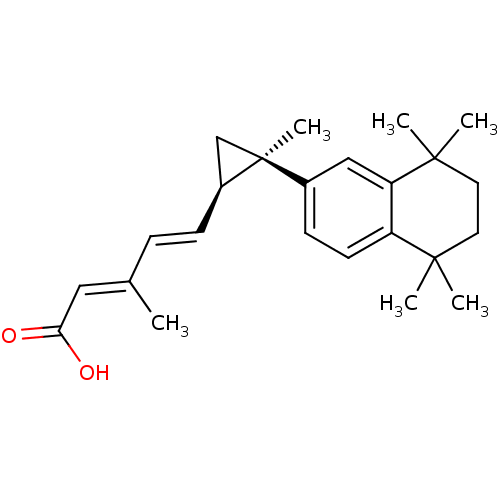
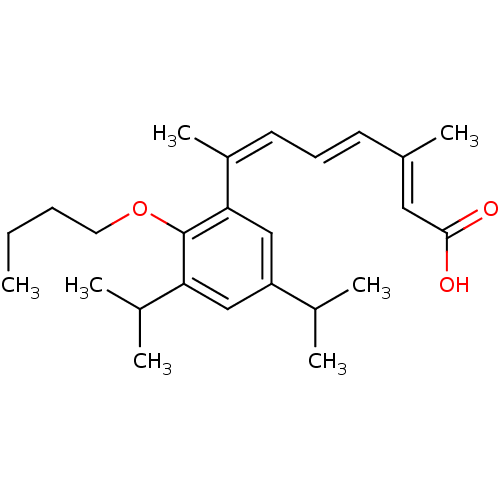
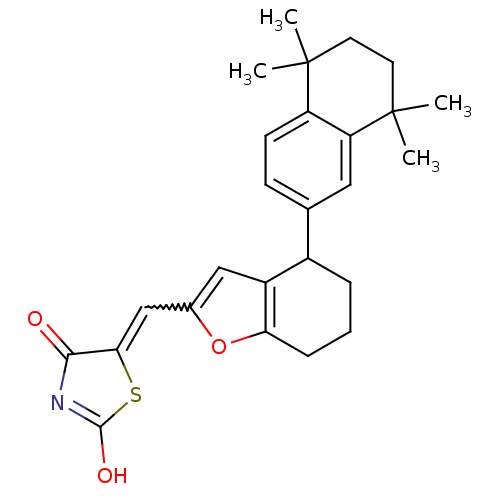
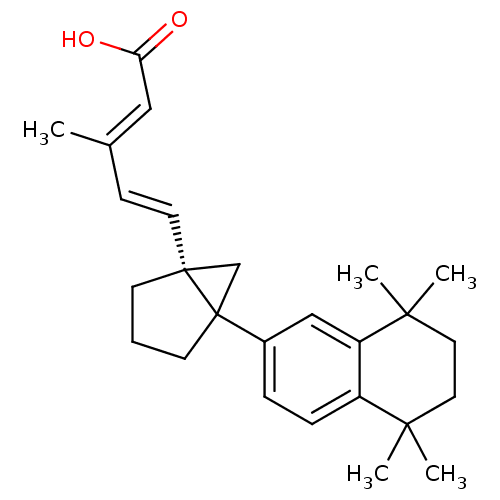
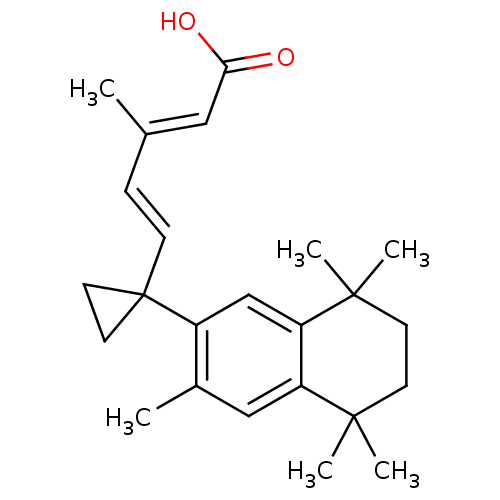
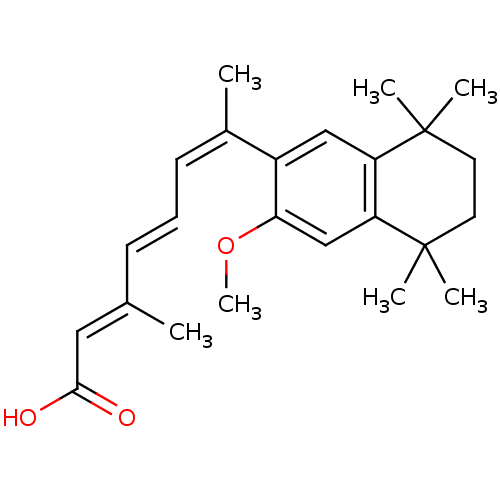
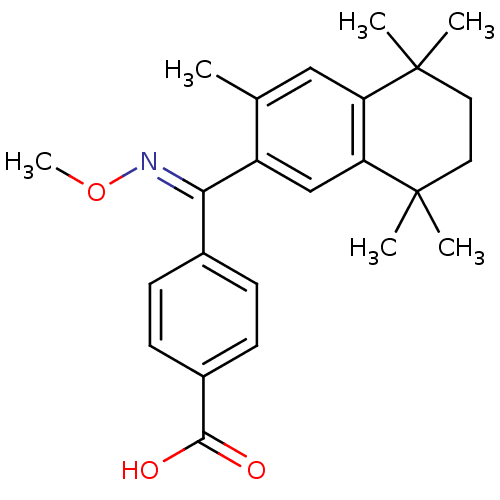
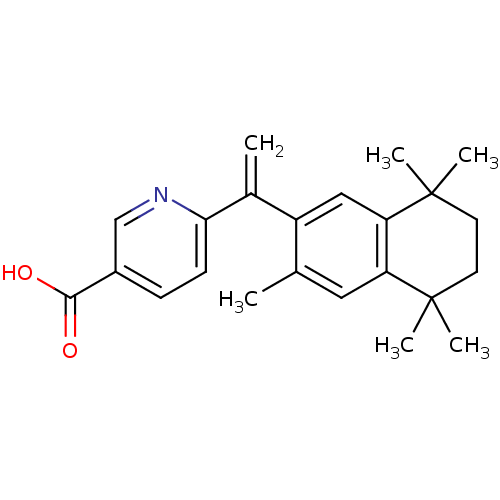
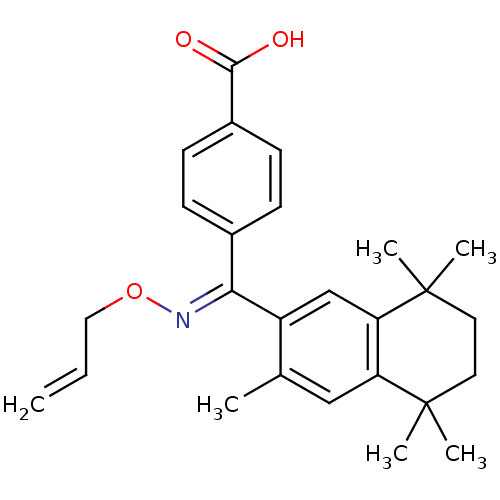
Affinity DataEC50: >0.00100nMAssay Description:Transcriptional activation of Retinoid X receptor RXR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: >0.00100nMAssay Description:Transcriptional activation of Retinoid X receptor RXR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: >0.00100nMAssay Description:Transcriptional activation of Retinoid X receptor RXR alphaMore data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: 0.130nMAssay Description:Agonist activity at RXR (unknown origin) in presence of RAR agonist Am80More data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha/RXR-beta/RXR-gamma(Homo sapiens (Human))
Tohoku Pharmaceutical University
Curated by ChEMBL
Tohoku Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: 0.140nMAssay Description:Agonist activity at RXR (unknown origin) in presence of RAR agonist Am80More data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 0.900nMAssay Description:Activity at RXRalpha by GAL4DNA cotransfection assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.900nMAssay Description:Activity at RXRalpha by GAL4DNA cotransfection assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1.40nMAssay Description:Activity at RXRalpha by GAL4DNA cotransfection assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1.5nMAssay Description:Compound was tested for functional activity in CV-1 cells transfected with an expression vector for retinoid X receptor alpha using transactivation a...More data for this Ligand-Target Pair
Affinity DataEC50: 1.5nMAssay Description:Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR alphaMore data for this Ligand-Target Pair
TargetRetinoic acid receptor RXR-alpha/Vitamin D3 receptor(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataEC50: 1.60nMAssay Description:Transactivation of recombinant human VDR/RXRalpha expressed in HEK293 cells after 24 hrs by Dual-luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1.80nMAssay Description:Activity at RXRalpha by GAL4DNA cotransfection assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1.80nMAssay Description:Activity at RXRalpha by GAL4DNA cotransfection assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1.80nMAssay Description:Activity at RXRalpha by GAL4DNA cotransfection assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2nMAssay Description:Inhibition of Retinoic acid receptor RXR-alpha agonist activity in vitroMore data for this Ligand-Target Pair
Affinity DataEC50: 2nMAssay Description:Effective concentration required for agonistic activity in CV-1 cells expressing RXR-alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 2nMAssay Description:Inhibition of Retinoic acid receptor RXR-alpha agonist activity in vitroMore data for this Ligand-Target Pair
Affinity DataEC50: 2nMAssay Description:Transcriptional ativation of Retinoid X receptor RXR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 2.10nMAssay Description:Activity at RXRalpha by GAL4DNA cotransfection assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2.30nMAssay Description:Activity at RXRalpha by GAL4DNA cotransfection assayMore data for this Ligand-Target Pair
Affinity DataEC50: 2.70nMAssay Description:Agonist activity at human RXRalpha LBD by cell based luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 3nMAssay Description:Effective concentration for Retinoid X receptor alpha activity in CV-1 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 3nMAssay Description:Effective concentration for Retinoid X receptor alpha activity in CV-1 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 3.30nMAssay Description:Antagonist activity against Retinoic acid receptor RXR-alpha expressed in CV-1 cell transcriptional activation assayMore data for this Ligand-Target Pair
Affinity DataEC50: 3.40nMAssay Description:Activity at RXRalpha by GAL4DNA cotransfection assayMore data for this Ligand-Target Pair
Affinity DataEC50: 3.40nMAssay Description:Antagonist activity against Retinoic acid receptor RXR-alpha expressed in CV-1 cell transcriptional activation assayMore data for this Ligand-Target Pair
Affinity DataEC50: 3.80nMAssay Description:Partial agonist activity at RXRalpha (unknown origin) expressed in COS1 cells after 18 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 4nMAssay Description:Agonist activity for retinoic acid receptor RXR alpha in transcriptional activation assayMore data for this Ligand-Target Pair
Affinity DataEC50: 4nMAssay Description:Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 4nMAssay Description:Effective concentration for antagonistic activity against RXR-alpha expressed in CV-1 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 4nMAssay Description:Ability to activate gene expression at Retinoic acid receptor RXR-alpha was evaluated in a cotransfection assay.More data for this Ligand-Target Pair
Affinity DataEC50: 4nMAssay Description:Effective concentration for lipogenesis induced by retinoid X receptor alpha in C3H10T1/2 clone 8 fibroblast cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 4nMAssay Description:Agonist activity at human RXR-alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 4nMAssay Description:Effective concentration for Retinoid X receptor alpha activity in CV-1 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 4nMAssay Description:Effective concentration for lipogenesis induced by retinoid X receptor alpha in C3H10T1/2 clone 8 fibroblast cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 4nMAssay Description:Effective potency in transcriptional activation assay in CV-1 cells expressing retinoic acid receptor RAR betaMore data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Transcriptional activity was evaluated in CV-1 cells transfected with expression vector for Retinoic acid receptor RXR-alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Transcriptional ativation of Retinoid X receptor RXR alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Transcriptional activity was evaluated in CV-1 cells transfected with expression vector for Retinoic acid receptor RXR-alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Effective concentration for Retinoid X receptor alpha activity in CV-1 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Effective concentration for lipogenesis induced by retinoid X receptor alpha in C3H10T1/2 clone 8 fibroblast cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 5nMAssay Description:Activity at RXRalpha by GAL4DNA cotransfection assayMore data for this Ligand-Target Pair
Affinity DataEC50: 5.28nMAssay Description:Agonist activity at RXRalpha by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 5.30nMAssay Description:Agonist activity at RXRalpha transfected in human COS1 cells after 18 hrs by luciferase reporter gene transactivation assayMore data for this Ligand-Target Pair
Affinity DataEC50: 6nMAssay Description:Ability to activate gene expression at Retinoic acid receptor RXR-alpha was evaluated in a cotransfection assay.More data for this Ligand-Target Pair
Affinity DataEC50: 6nMAssay Description:Agonist activity at human RXR-alpha expressed in U2OS cells by PathHunter assayMore data for this Ligand-Target Pair
Affinity DataEC50: 6nMAssay Description:Transcriptional activity was evaluated in CV-1 cells transfected with expression vector for Retinoic acid receptor RXR-alphaMore data for this Ligand-Target Pair
Affinity DataEC50: 6nMAssay Description:Effective concentration against Retinoic acid receptor RXR-alphaMore data for this Ligand-Target Pair

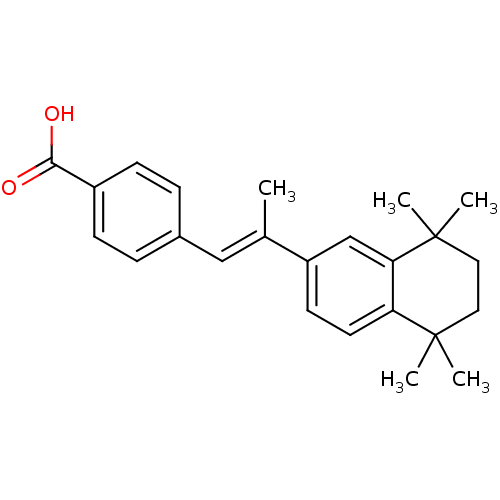
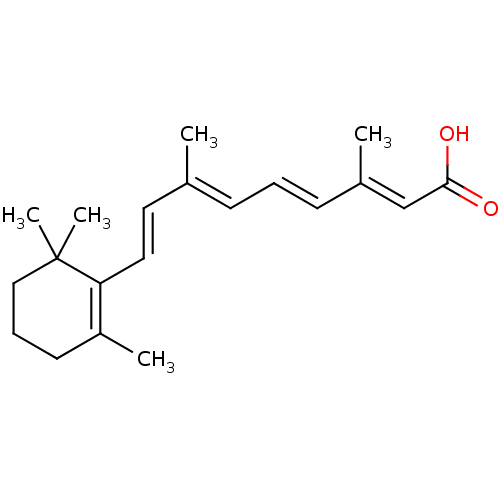
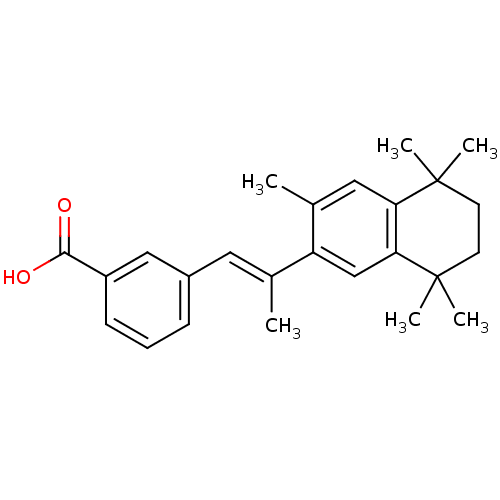
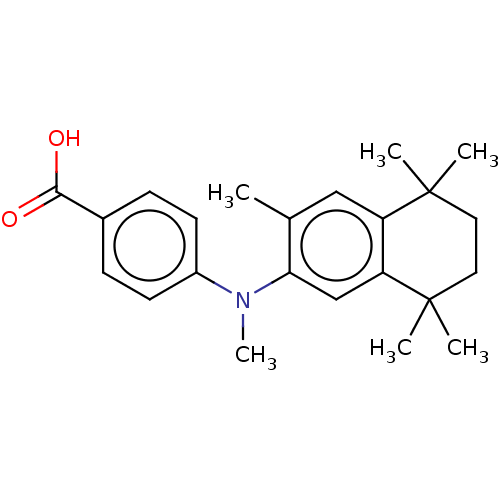
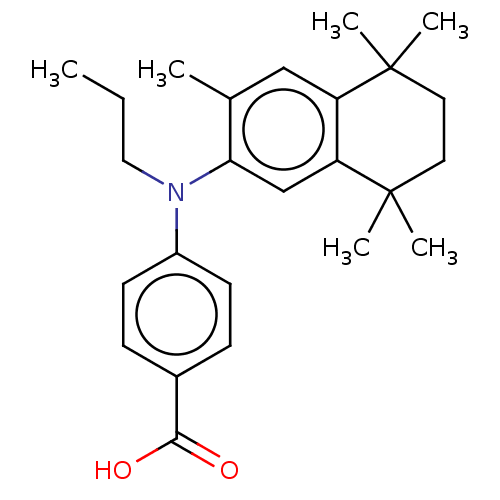

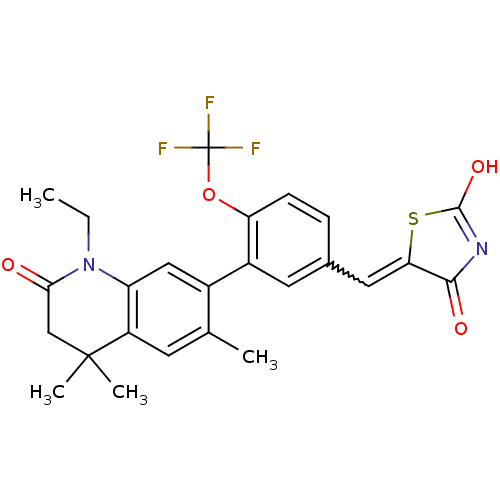
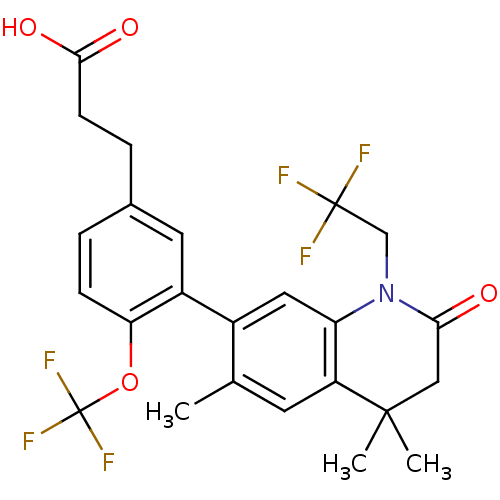
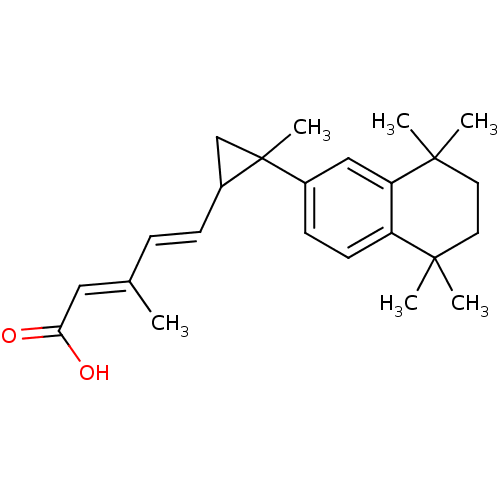
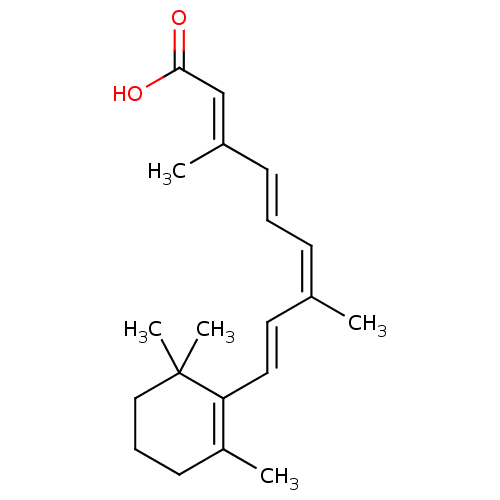
 3D Structure (crystal)
3D Structure (crystal)