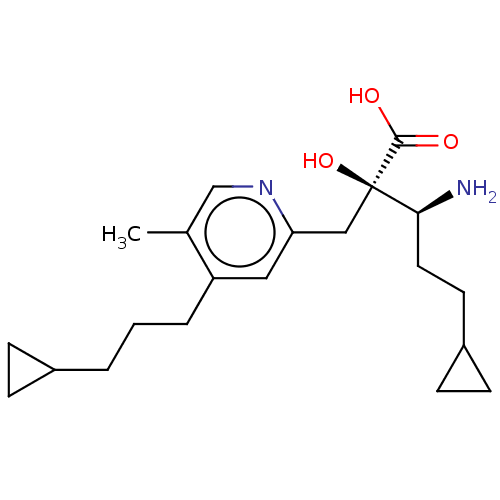
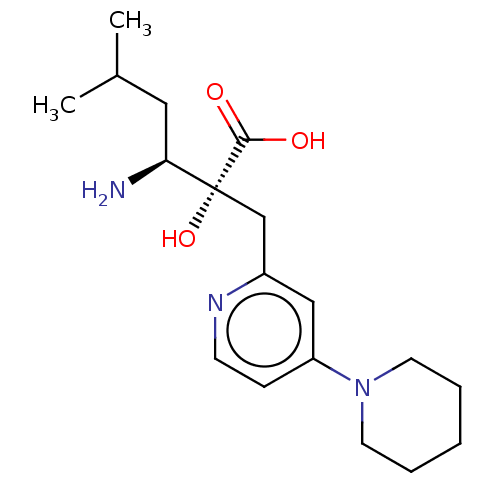
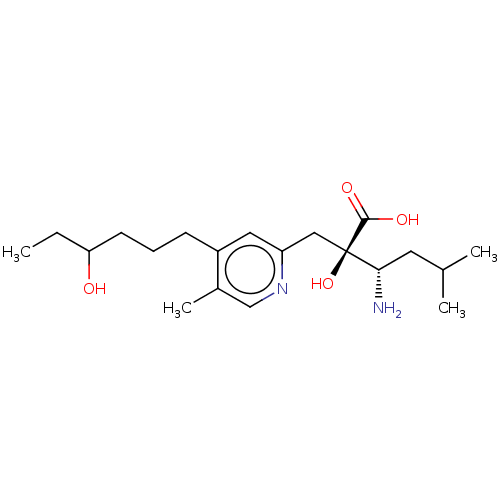
Affinity DataIC50: 0.420nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
Affinity DataIC50: 0.420nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.560nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.580nMAssay Description:The inventors have assumed that inhibition of nocturnal activity of placental leucine aminopeptidase (P-LAP), i.e. aminopeptidase that cleaves AVP, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.620nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
Affinity DataIC50: 0.620nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.650nMAssay Description:The inventors have assumed that inhibition of nocturnal activity of placental leucine aminopeptidase (P-LAP), i.e. aminopeptidase that cleaves AVP, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.770nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
Affinity DataIC50: 0.770nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
Affinity DataIC50: 0.770nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.770nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.890nMAssay Description:hP-LAP: HEK293 cells forced to transiently express hP-LAP were prepared by lipofection, homogenized, and then subjected to ultracentrifugation at 100...More data for this Ligand-Target Pair
Affinity DataIC50: 0.890nMAssay Description:HEK293 cells forced to transiently express hP-LAP (J Biol Chem 1996; 271: 56-61) were prepared by lipofection, homogenized, and then subjected to ult...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.910nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.910nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
Affinity DataIC50: 0.920nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.920nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
Affinity DataIC50: 0.940nMAssay Description:hP-LAP: HEK293 cells forced to transiently express hP-LAP were prepared by lipofection, homogenized, and then subjected to ultracentrifugation at 100...More data for this Ligand-Target Pair
Affinity DataIC50: 0.940nMAssay Description:HEK293 cells forced to transiently express hP-LAP (J Biol Chem 1996; 271: 56-61) were prepared by lipofection, homogenized, and then subjected to ult...More data for this Ligand-Target Pair
Affinity DataIC50: 0.940nMAssay Description:The inventors have assumed that inhibition of nocturnal activity of placental leucine aminopeptidase (P-LAP), i.e. aminopeptidase that cleaves AVP, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.950nMAssay Description:hP-LAP: HEK293 cells forced to transiently express hP-LAP were prepared by lipofection, homogenized, and then subjected to ultracentrifugation at 100...More data for this Ligand-Target Pair
Affinity DataIC50: 0.950nMAssay Description:HEK293 cells forced to transiently express hP-LAP (J Biol Chem 1996; 271: 56-61) were prepared by lipofection, homogenized, and then subjected to ult...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:hP-LAP: HEK293 cells forced to transiently express hP-LAP were prepared by lipofection, homogenized, and then subjected to ultracentrifugation at 100...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:HEK293 cells forced to transiently express hP-LAP (J Biol Chem 1996; 271: 56-61) were prepared by lipofection, homogenized, and then subjected to ult...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:The inventors have assumed that inhibition of nocturnal activity of placental leucine aminopeptidase (P-LAP), i.e. aminopeptidase that cleaves AVP, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:HEK293 cells forced to transiently express hP-LAP (J Biol Chem 1996; 271: 56-61) were prepared by lipofection, homogenized, and then subjected to ult...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:hP-LAP: HEK293 cells forced to transiently express hP-LAP were prepared by lipofection, homogenized, and then subjected to ultracentrifugation at 100...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:hP-LAP: HEK293 cells forced to transiently express hP-LAP were prepared by lipofection, homogenized, and then subjected to ultracentrifugation at 100...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:HEK293 cells forced to transiently express hP-LAP (J Biol Chem 1996; 271: 56-61) were prepared by lipofection, homogenized, and then subjected to ult...More data for this Ligand-Target Pair