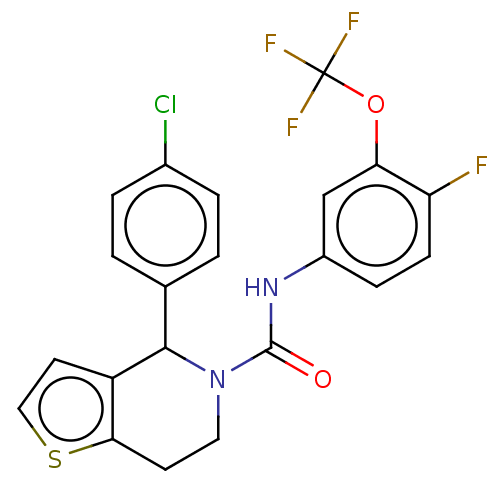
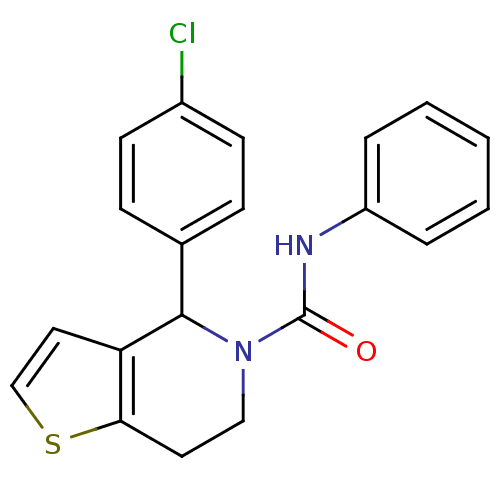
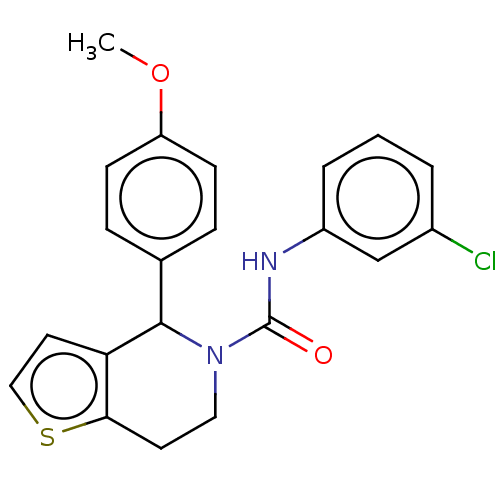
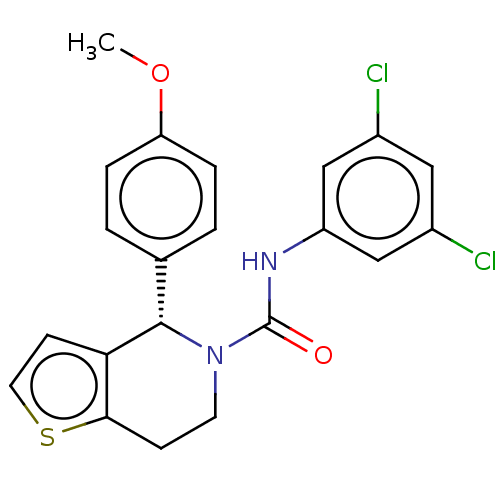
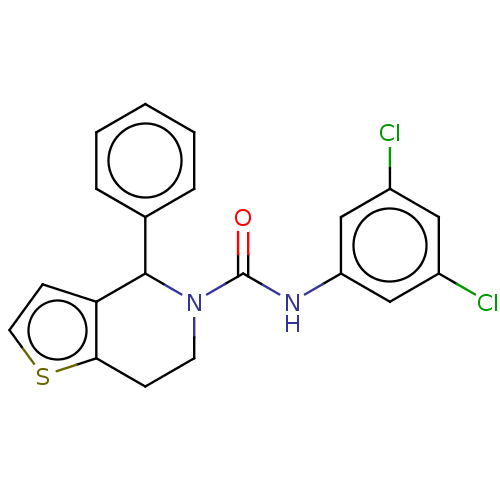
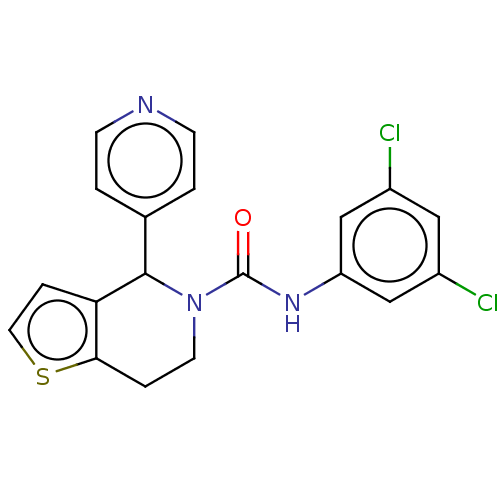
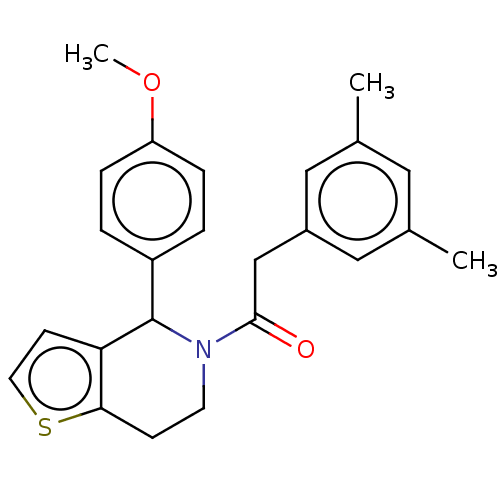
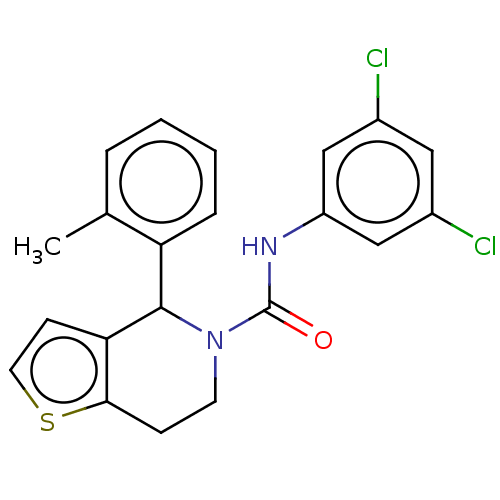
Affinity DataIC50: 23nMAssay Description:Antagonist activity at rat luteinizing hormone receptor expressed in rat ovary granulosa GLHR15 cell suspension assessed as reduction in agonist-indu...More data for this Ligand-Target Pair
Affinity DataIC50: 46nMAssay Description:Antagonist activity at rat luteinizing hormone receptor expressed in rat ovary granulosa GLHR15 cell suspension assessed as reduction in agonist-indu...More data for this Ligand-Target Pair
Affinity DataIC50: 81nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 95nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 96nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 97nMAssay Description:Negative allosteric modulation of human luteinizing hormone receptor assessed as reduction in glycoprotein human luteinizing hormone-induced agonist ...More data for this Ligand-Target Pair
Affinity DataIC50: 102nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 107nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 127nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 174nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 185nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 193nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Negative allosteric modulation of human luteinizing hormone receptor assessed as reduction in glycoprotein human luteinizing hormone-induced agonist ...More data for this Ligand-Target Pair
Affinity DataIC50: 207nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 212nMAssay Description:Antagonist activity at rat luteinizing hormone receptor expressed in rat ovary granulosa GLHR15 cell suspension assessed as reduction in agonist-indu...More data for this Ligand-Target Pair
Affinity DataIC50: 275nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 312nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 332nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 378nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 403nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 412nMAssay Description:Antagonist activity at rat luteinizing hormone receptor expressed in rat ovary granulosa GLHR15 cell suspension assessed as reduction in agonist-indu...More data for this Ligand-Target Pair
Affinity DataIC50: 486nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 580nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 650nMAssay Description:Negative allosteric modulation of human luteinizing hormone receptor assessed as reduction in Org 43553-induced agonist activity preincubated for 20 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Negative allosteric modulation of human luteinizing hormone receptor assessed as reduction in Org 43553-induced agonist activity preincubated for 20 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Negative allosteric modulation of human luteinizing hormone receptor assessed as reduction in Org 43553-induced agonist activity preincubated for 20 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Negative allosteric modulation of human luteinizing hormone receptor assessed as reduction in Org 43553-induced agonist activity preincubated for 20 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.62E+3nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.68E+3nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Negative allosteric modulation of human luteinizing hormone receptor assessed as reduction in Org 43553-induced agonist activity preincubated for 20 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.85E+3nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Negative allosteric modulation of human luteinizing hormone receptor assessed as reduction in glycoprotein human luteinizing hormone-induced agonist ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Negative allosteric modulation of human luteinizing hormone receptor assessed as reduction in glycoprotein human luteinizing hormone-induced agonist ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30E+3nMAssay Description:Displacement of [3H]Org43553 from human luteinizing hormone receptor expressed in CHOK1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.31E+3nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+3nMAssay Description:Negative allosteric modulation of human luteinizing hormone receptor assessed as reduction in glycoprotein human luteinizing hormone-induced agonist ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.61E+3nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.13E+3nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.24E+3nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.32E+3nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.65E+3nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.89E+3nMAssay Description:Antagonist activity at rat luteinizing hormone receptor expressed in rat ovary granulosa GLHR15 cell suspension assessed as reduction in agonist-indu...More data for this Ligand-Target Pair
Affinity DataIC50: 5.18E+3nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.79E+3nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: 7.47E+3nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.60E+4nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.65E+4nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.65E+4nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.65E+4nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.65E+4nMAssay Description:Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ...More data for this Ligand-Target Pair