TargetLysine-specific histone demethylase 1B(Mus musculus)
Key Laboratory Of Henan Provinc
Curated by ChEMBL
Key Laboratory Of Henan Provinc
Curated by ChEMBL
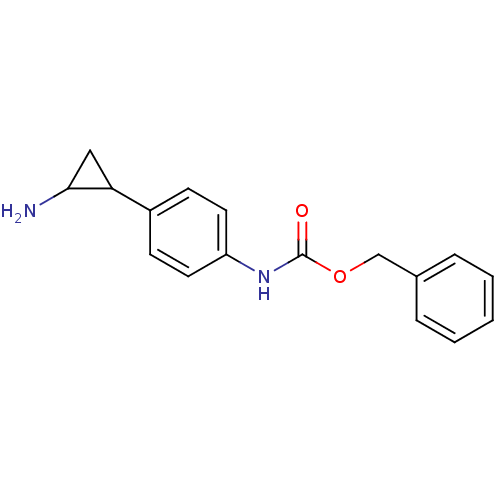
Affinity DataKi: 2.00E+4nMAssay Description:Inhibition of recombinant mouse LSD2 expressed in Escherichia coli using histone H3 peptide dimethylated at Lys4 as substrate by peroxidase-coupled a...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1B(Mus musculus)
Key Laboratory Of Henan Provinc
Curated by ChEMBL
Key Laboratory Of Henan Provinc
Curated by ChEMBL
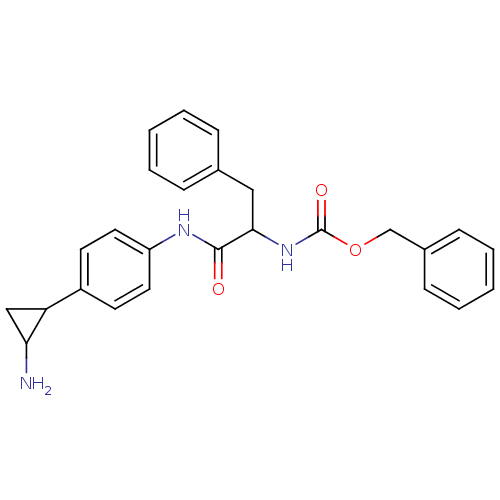
Affinity DataKi: 3.80E+4nMAssay Description:Inhibition of recombinant mouse LSD2 expressed in Escherichia coli using histone H3 peptide dimethylated at Lys4 as substrate by peroxidase-coupled a...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1B(Mus musculus)
Key Laboratory Of Henan Provinc
Curated by ChEMBL
Key Laboratory Of Henan Provinc
Curated by ChEMBL
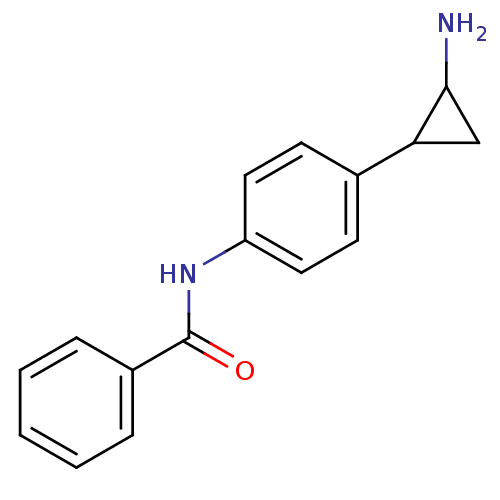
Affinity DataKi: 6.10E+4nMAssay Description:Inhibition of recombinant mouse LSD2 expressed in Escherichia coli using histone H3 peptide dimethylated at Lys4 as substrate by peroxidase-coupled a...More data for this Ligand-Target Pair