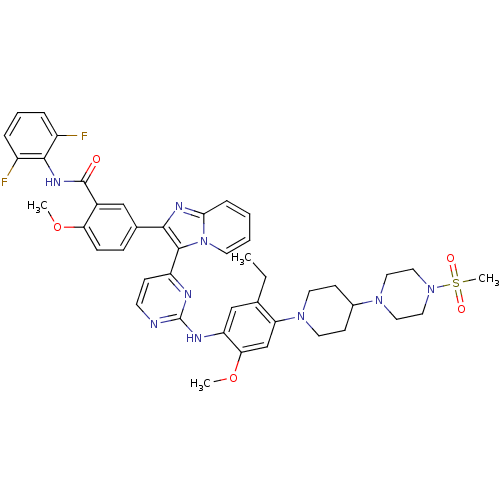
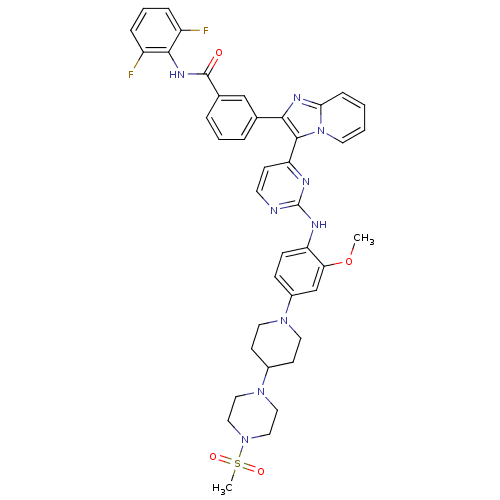
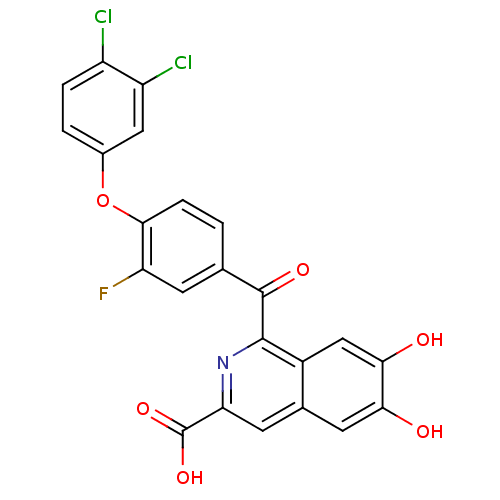
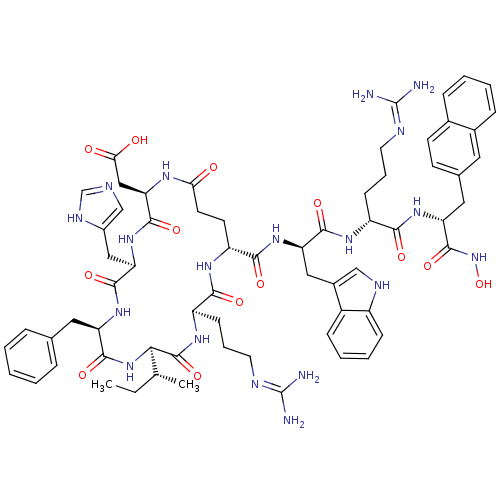
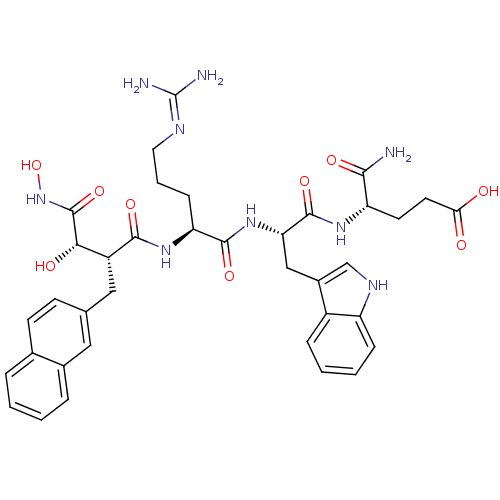
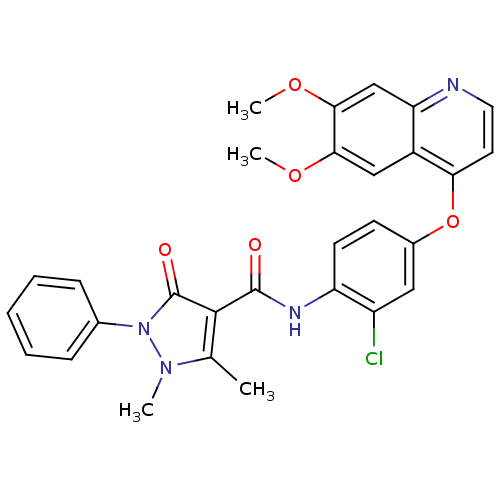
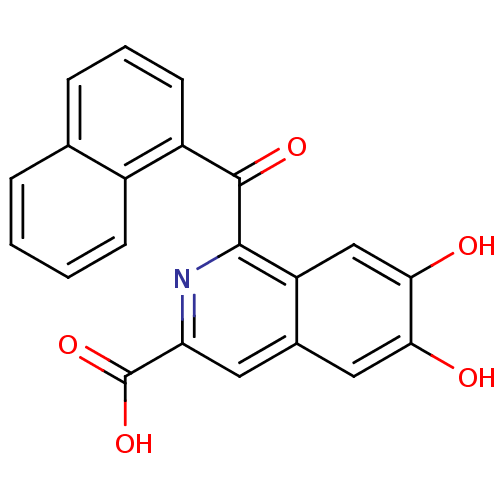
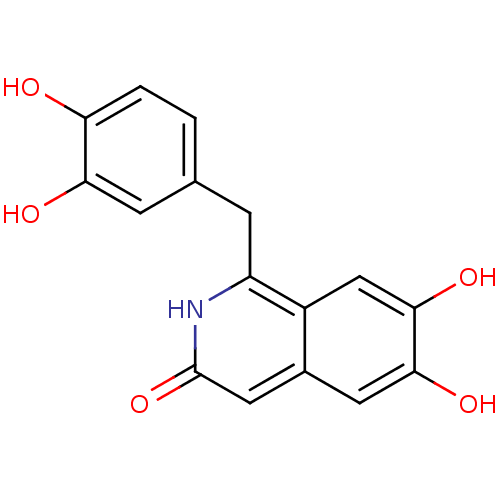
TargetInsulin-like growth factor-binding protein 4(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:The compound was tested for binding affinity against Insulin-like growth factor binding protein 4More data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Binding affinity to insulin receptor by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Inhibition of human recombinant IDE-mediated fluorescein-Abeta-(1-40)-Lys-biotin degradationMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Binding affinity to IGF1R by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Inhibition of human recombinant IDE expressed in CHO cells in presence of [125I]-insulin by HTRF assayMore data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 2(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 2.10nMAssay Description:The compound was tested for binding affinity against insulin-like growth factor binding protein 2More data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Binding affinity to insulin receptor by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Inhibition of human recombinant IDE-mediated FRET1 degradationMore data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 1(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 5.10nMAssay Description:The compound was tested for binding affinity against insulin-like growth factor binding protein 1More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 5(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 5.20nMAssay Description:The compound was tested for binding affinity against Insulin-like growth factor binding protein 5More data for this Ligand-Target Pair
Affinity DataKi: 5.20nMAssay Description:Binding affinity to IGF1R by liquid scintillation countingMore data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 5.60nMAssay Description:Ability to displace Insulin-Like Growth Factor (IGF-I) from its binding to human insulin-like growth factor binding protein 3 (hIGFBP-3)More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 5(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 5.60nMAssay Description:Ability of compound to displace Insulin-Like Growth Factor (IGF-I) from its binding to Insulin-like growth factor binding protein 5 (IGFBP-5)More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 5.60nMAssay Description:Inhibitory activity against Insulin-like growth factor binding protein 3More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 4(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 6.20nMAssay Description:The compound was tested for binding affinity against Insulin-like growth factor binding protein 4More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 5(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 7.20nMAssay Description:Ability of compound to displace Insulin-Like Growth Factor (IGF-I) from its binding to Insulin-like growth factor binding protein 5 (IGFBP-5)More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 7.5nMAssay Description:Inhibitory activity against Insulin-like growth factor binding protein 3More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Ability of compound to displace Insulin-Like Growth Factor (IGF-I) from its binding to Insulin-like growth factor binding protein 3 (IGFBP-3)More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Ability to displace Insulin-Like Growth Factor (IGF-I) from its binding to human insulin-like growth factor binding protein 3 (hIGFBP-3)More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 9.40nMAssay Description:Ability to displace Insulin-Like Growth Factor (IGF-I) from its binding to human insulin-like growth factor binding protein 3 (hIGFBP-3)More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Ability to displace Insulin-Like Growth Factor (IGF-I) from its binding to human insulin-like growth factor binding protein 3 (hIGFBP-3)More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Ability to displace Insulin-Like Growth Factor (IGF-I) from its binding to human insulin-like growth factor binding protein 3 (hIGFBP-3)More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Ability to displace Insulin-Like Growth Factor (IGF-I) from its binding to human insulin-like growth factor binding protein 3 (hIGFBP-3)More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Inhibitory activity against Insulin-like growth factor binding protein 3More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 5(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Ability of compound to displace Insulin-Like Growth Factor (IGF-I) from its binding to Insulin-like growth factor binding protein 5 (IGFBP-5)More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 2(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 17nMAssay Description:The compound was tested for binding affinity against insulin-like growth factor binding protein 2More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 18nMAssay Description:Ability to displace Insulin-Like Growth Factor (IGF-I) from its binding to human insulin-like growth factor binding protein 3 (hIGFBP-3)More data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Inhibition of IGF1R by HTRF assayMore data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Ability to displace Insulin-Like Growth Factor (IGF-I) from its binding to human insulin-like growth factor binding protein 3 (hIGFBP-3)More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 5(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 21nMAssay Description:The compound was tested for binding affinity against Insulin-like growth factor binding protein 5More data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Inhibition of human recombinant IDE-mediated fluorescein-Abeta-(1-40)-Lys-biotin degradationMore data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Inhibition of human recombinant IDE-mediated FRET1 degradationMore data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 24nMAssay Description:Ability to displace Insulin-Like Growth Factor (IGF-I) from its binding to human insulin-like growth factor binding protein 3 (hIGFBP-3)More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 6(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 24nMAssay Description:The compound was tested for binding affinity against Insulin-like growth factor binding protein 6More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 25nMAssay Description:Ability to displace Insulin-Like Growth Factor (IGF-I) from its binding to human insulin-like growth factor binding protein 3 (hIGFBP-3)More data for this Ligand-Target Pair
Affinity DataKi: 25nMAssay Description:Inhibition of IGF1R by HTRF assayMore data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Inhibition of human recombinant IDE-mediated FRET1 degradationMore data for this Ligand-Target Pair
Affinity DataKi: 27nMAssay Description:Inhibition of IGF1R by HTRF assayMore data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 27nMAssay Description:Inhibitory activity against Insulin-like growth factor binding protein 3More data for this Ligand-Target Pair
Affinity DataKi: 27nMAssay Description:Inhibition of IGF1R by HTRF assayMore data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 28nMAssay Description:Inhibitory activity against Insulin-like growth factor binding protein 3More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 29nMAssay Description:Ability to displace Insulin-Like Growth Factor (IGF-I) from its binding to human insulin-like growth factor binding protein 3 (hIGFBP-3)More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 29nMAssay Description:Inhibitory activity against Insulin-like growth factor binding protein 3More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 31nMAssay Description:Ability to displace Insulin-Like Growth Factor (IGF-I) from its binding to human insulin-like growth factor binding protein 3 (hIGFBP-3)More data for this Ligand-Target Pair
Affinity DataKi: 31nMAssay Description:Inhibition of IGF1R by HTRF assayMore data for this Ligand-Target Pair
Affinity DataKi: 32nMAssay Description:Inhibition of IGF1R by HTRF assayMore data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 32nMAssay Description:Ability to displace Insulin-Like Growth Factor (IGF-I) from its binding to human insulin-like growth factor binding protein 3 (hIGFBP-3)More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 32nMAssay Description:Ability to displace Insulin-Like Growth Factor (IGF-I) from its binding to human insulin-like growth factor binding protein 3 (hIGFBP-3)More data for this Ligand-Target Pair
TargetInsulin-like growth factor-binding protein 3(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 32nMAssay Description:Ability to displace Insulin-Like Growth Factor (IGF-I) from its binding to human insulin-like growth factor binding protein 3 (hIGFBP-3)More data for this Ligand-Target Pair