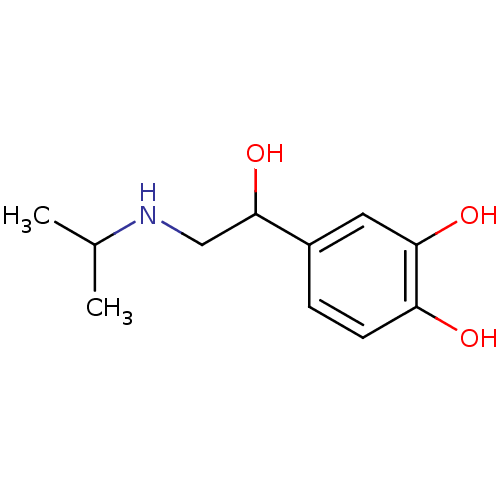
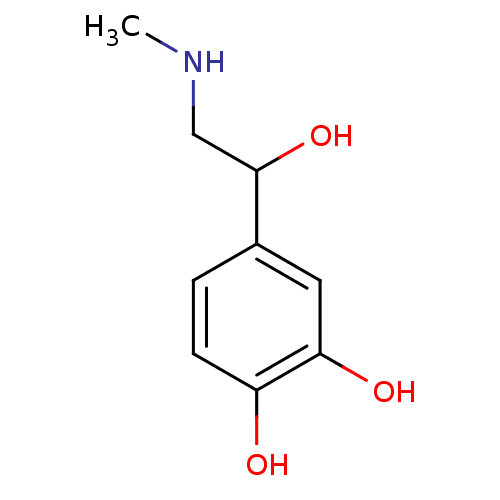
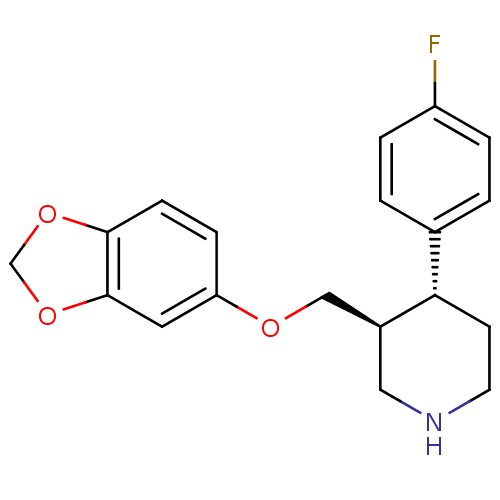
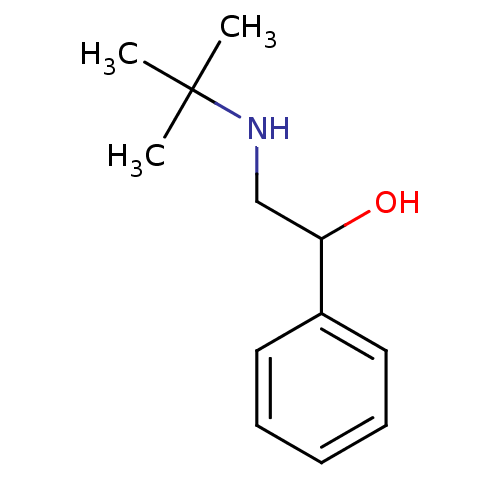
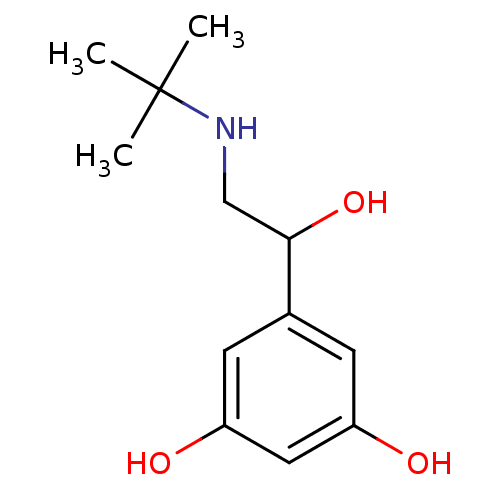
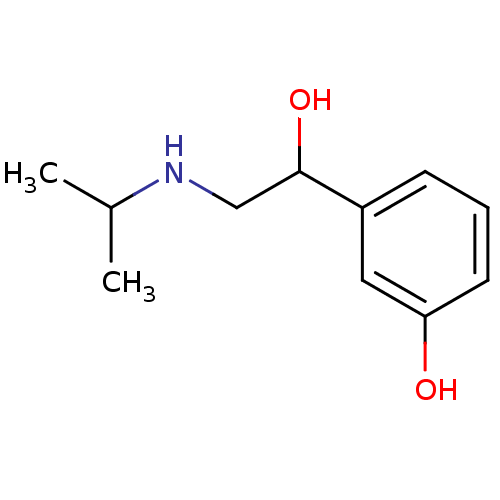
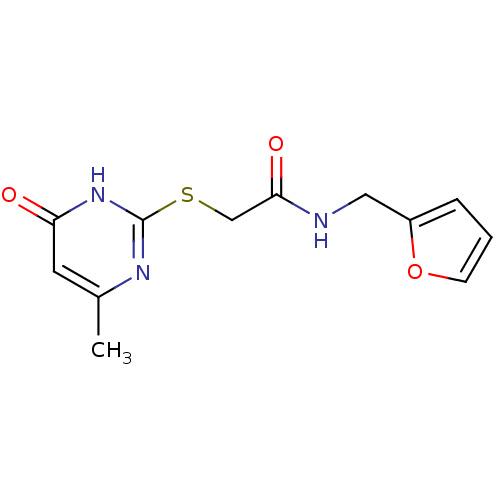
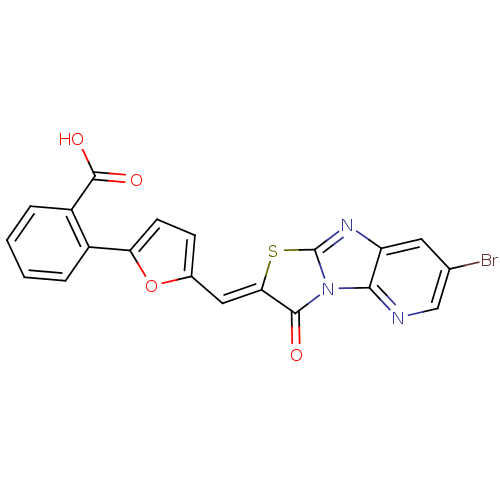
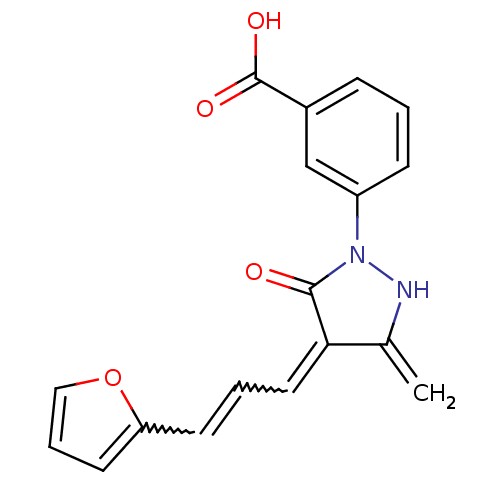
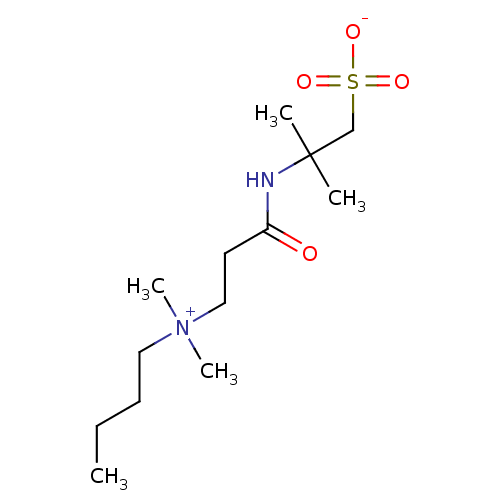
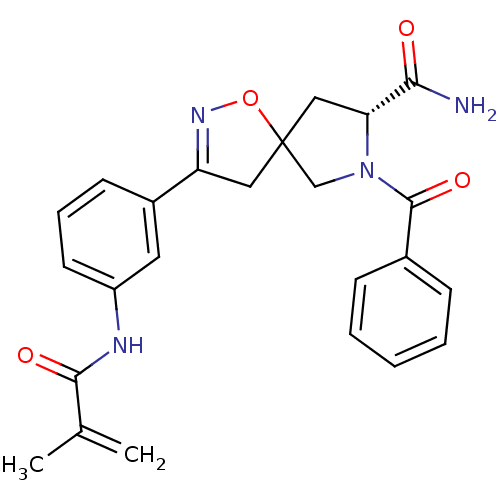
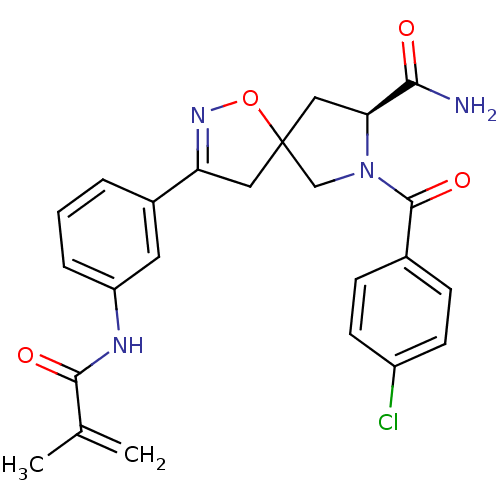
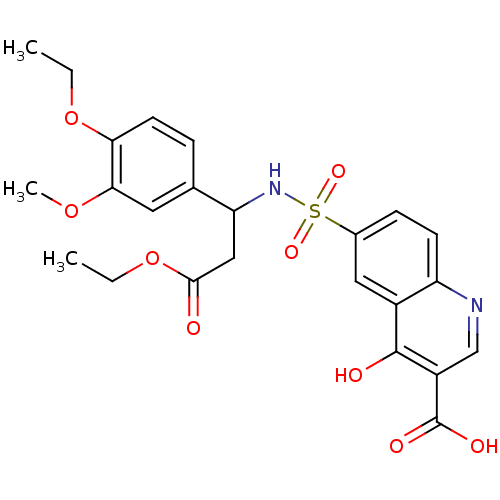
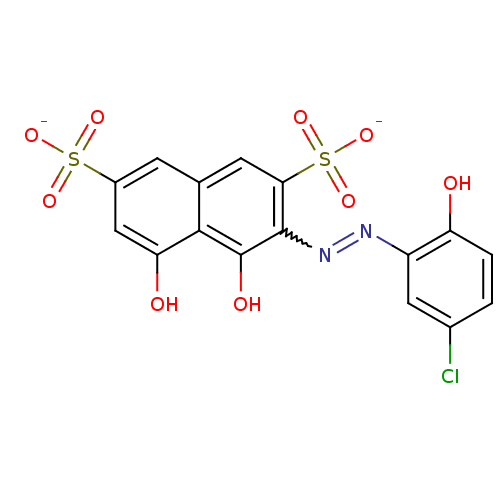
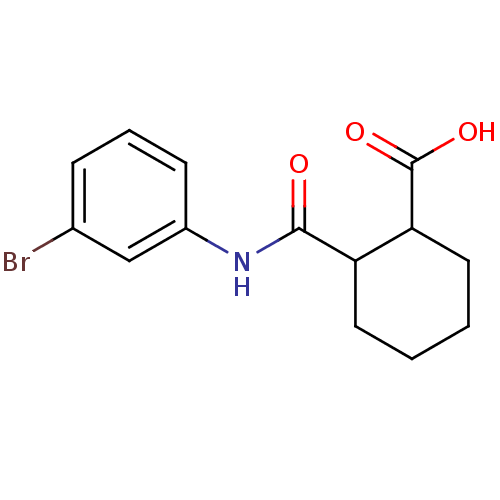
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
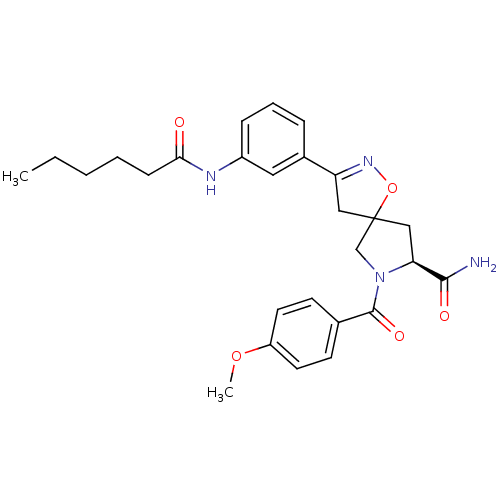
Affinity DataKd: 2nMAssay Description:Binding affinity to full length human GRK2 by Thermofluor thermal shift assayMore data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
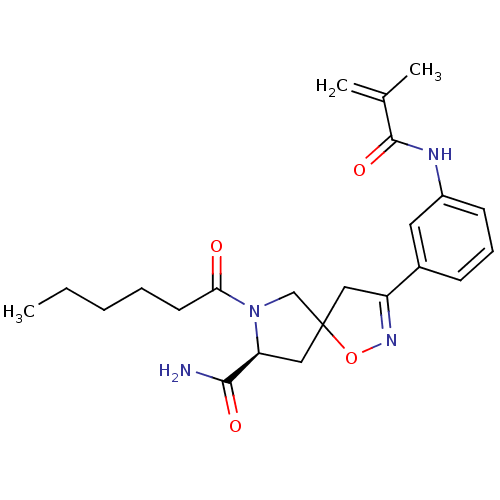
Affinity DataKd: 200nMAssay Description:The binding of ligands to wild-type beta-AR was measured by competition with 35 pM 125I-CYP.More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
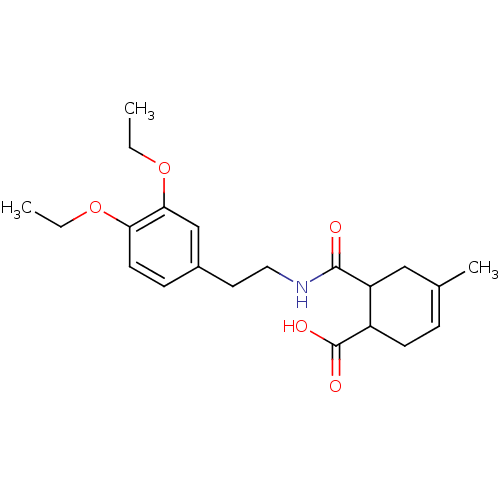
Affinity DataKd: 610nMAssay Description:The binding of ligands to wild-type beta-AR was measured by competition with 35 pM 125I-CYP.More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
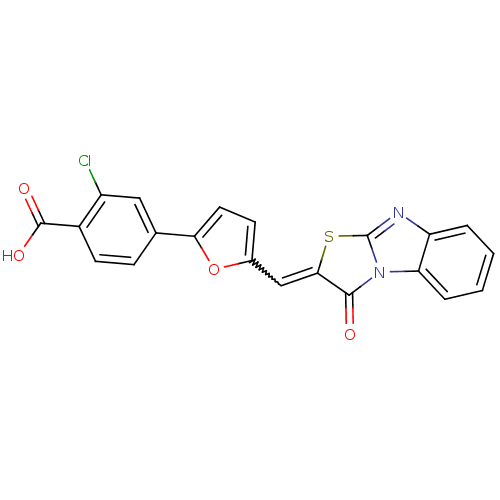
Affinity DataKd: 2.98E+3nMAssay Description:Binding affinity to full length human GRK2 by Thermofluor thermal shift assayMore data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataKd: 3.33E+3nMAssay Description:Binding affinity to full length human GRK2 by Thermofluor thermal shift assayMore data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataKd: 4.10E+3nMAssay Description:The binding of ligands to wild-type beta-AR was measured by competition with 35 pM 125I-CYP.More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataKd: 5.20E+3nMAssay Description:The binding of ligands to wild-type beta-AR was measured by competition with 35 pM 125I-CYP.More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataKd: 7.40E+3nMAssay Description:The binding of ligands to wild-type beta-AR was measured by competition with 35 pM 125I-CYP.More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataKd: 1.30E+4nMAssay Description:The binding of ligands to wild-type beta-AR was measured by competition with 35 pM 125I-CYP.More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataKd: 3.00E+4nMAssay Description:The binding of ligands to wild-type beta-AR was measured by competition with 35 pM 125I-CYP.More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataKd: 9.00E+5nMAssay Description:The binding of ligands to wild-type beta-AR was measured by competition with 35 pM 125I-CYP.More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataKi: 6.70E+4nMAssay Description:Inhibition of beta-adrenergic receptor kinase(unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+6nMAssay Description:Inhibition of bovine cerebral cortex GRK2 assessed as decrease in phosphorylation of beta-adrenoceptor in presence of [gamma-32P]-ATP by SDS-PAGE ana...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 2.21E+4nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 2.21E+4nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 3.50E+3nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 2.39E+3nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 70nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 1.48E+4nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 4.53E+3nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 670nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 3.09E+4nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 1.21E+3nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 1.94E+4nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 60nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 3.62E+4nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 120nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 9.42E+3nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 3.33E+4nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 1.25E+4nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 1.06E+4nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 1.94E+3nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 730nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 4.38E+3nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 2.13E+4nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 110nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 8.59E+4nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 3.05E+3nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 7.83E+3nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 5.13E+4nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 1.78E+4nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 5.83E+4nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 5.01E+3nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 9.71E+3nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 3.62E+3nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 1.63E+4nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 1.11E+4nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 7.53E+3nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 720nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair
TargetBeta-adrenergic receptor kinase 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataEC50: 3.85E+3nMAssay Description:University of New Mexico Assay Overview: Assay Support: 1 R03 DA030557-01A1 Project Title: RNA Aptamer-based screen for Selective Inhibitors of ...More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)