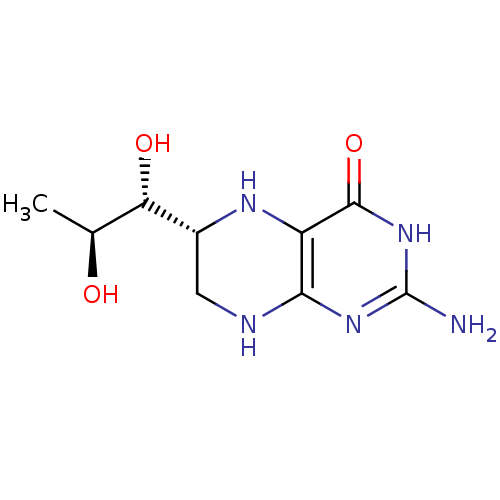
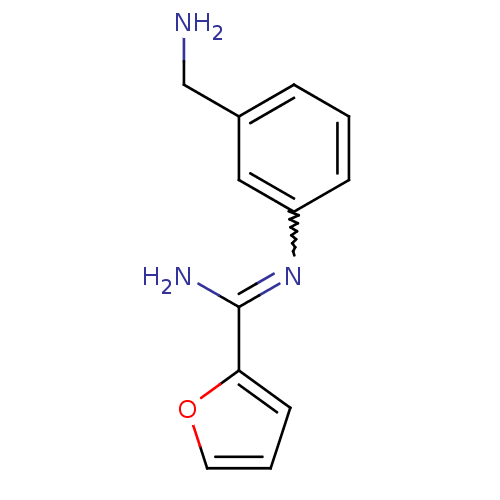
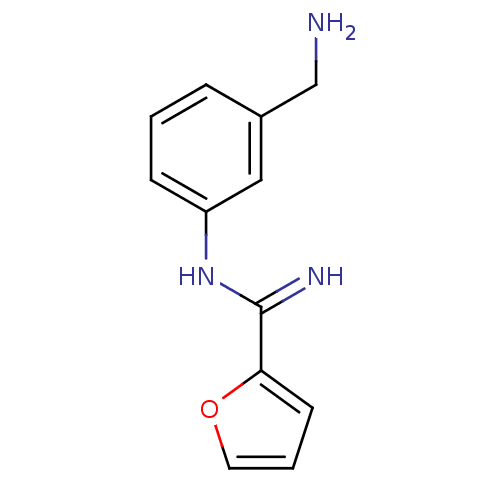
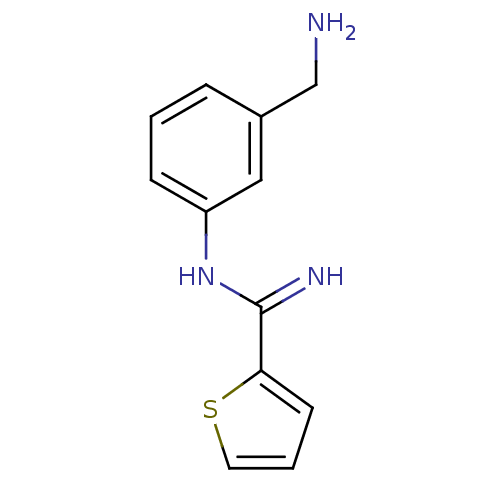
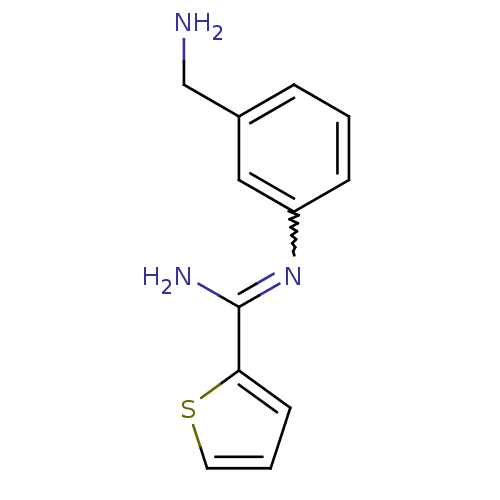
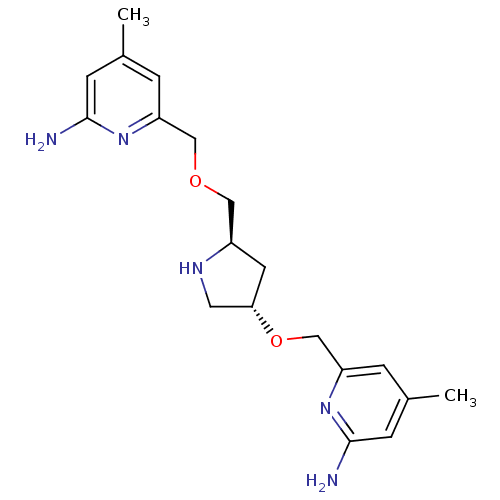
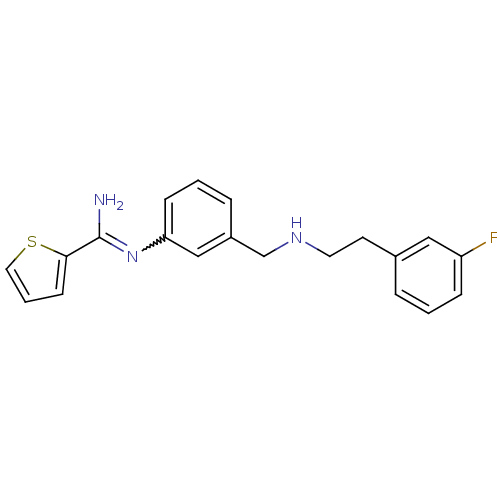
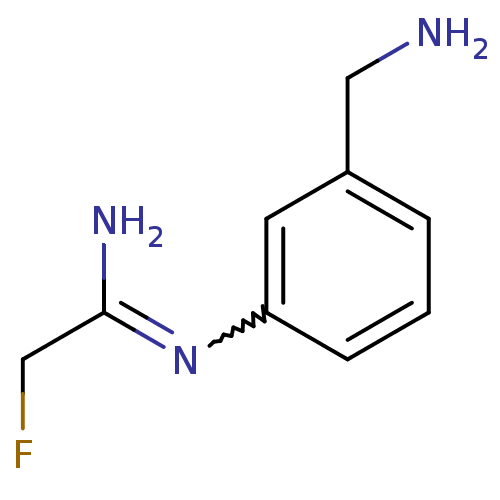
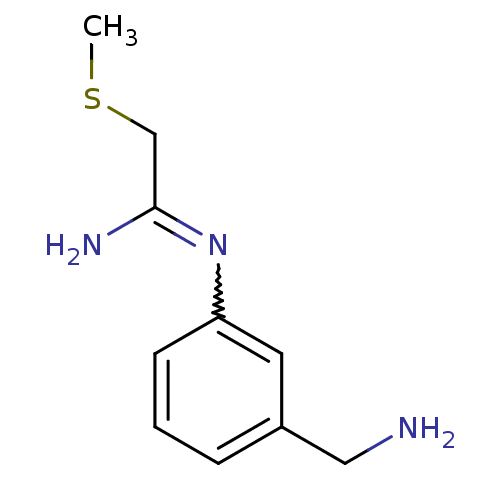
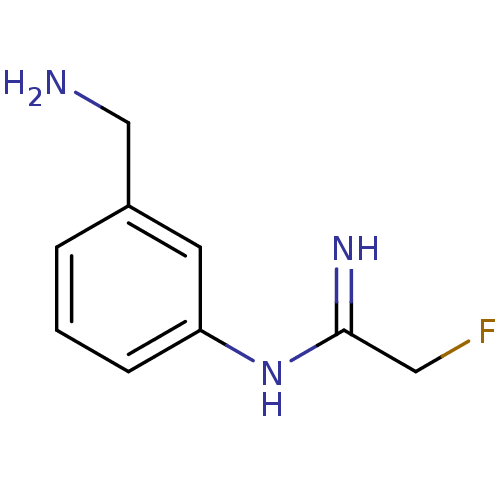
Affinity DataKd: 30nMpH: 7.5 T: 2°CAssay Description:The buffer contained 50 mM HEPES (pH 7.5), 10% glycerol, and 100 mM NaCl, containing 1 mM imidazole. The spectral shift was monitored for a few minut...More data for this Ligand-Target Pair
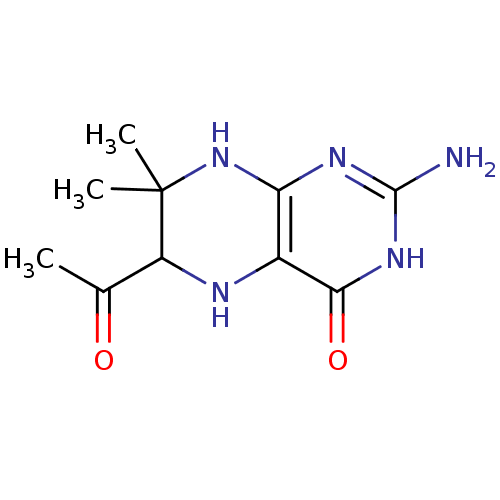
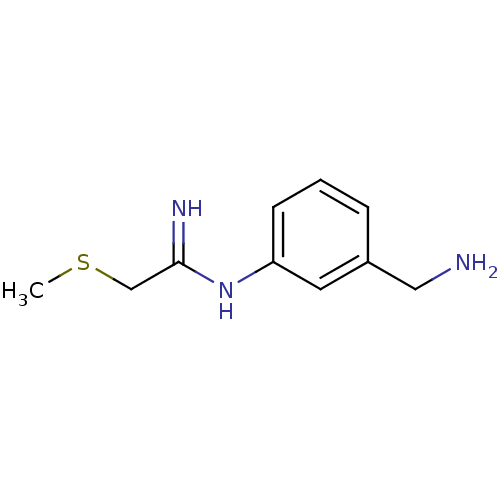
Affinity DataKd: 50nMpH: 7.5 T: 2°CAssay Description:The buffer contained 50 mM HEPES (pH 7.5), 10% glycerol, and 100 mM NaCl, containing 1 mM imidazole. The spectral shift was monitored for a few minut...More data for this Ligand-Target Pair
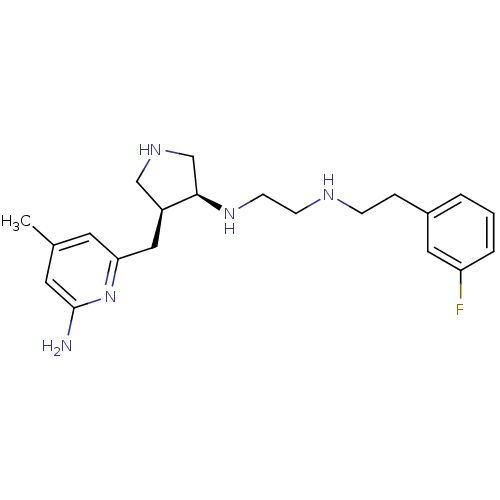
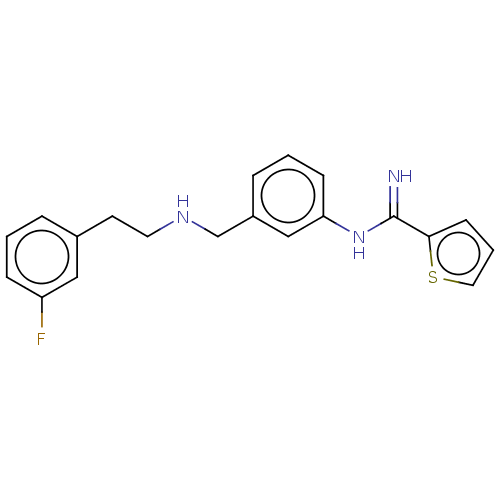
Affinity DataKd: 198nMpH: 7.5 T: 2°CAssay Description:The buffer contained 50 mM HEPES (pH 7.5), 10% glycerol, and 100 mM NaCl, containing 1 mM imidazole. The spectral shift was monitored for a few minut...More data for this Ligand-Target Pair
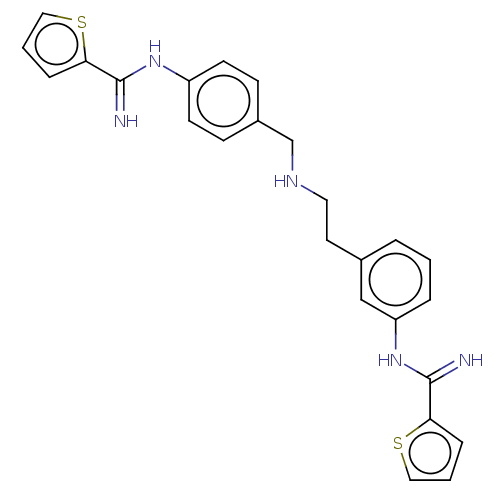
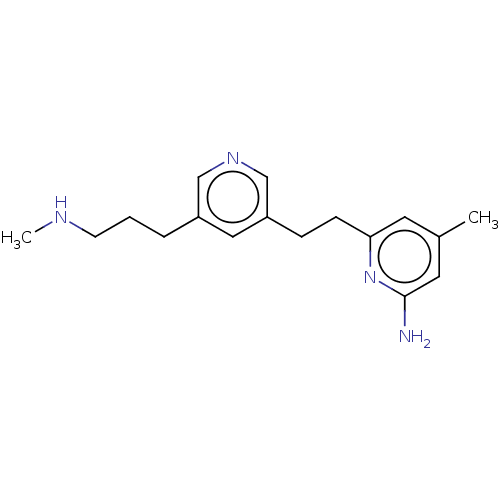
Affinity DataKd: 212nMpH: 7.5 T: 2°CAssay Description:The buffer contained 50 mM HEPES (pH 7.5), 10% glycerol, and 100 mM NaCl, containing 1 mM imidazole. The spectral shift was monitored for a few minut...More data for this Ligand-Target Pair
Affinity DataKd: 1.12E+3nMAssay Description:Activity of tetrahydrobiopterin free nNOSMore data for this Ligand-Target Pair
Affinity DataKd: 1.51E+5nMAssay Description:Activity of tetrahydrobiopterin free nNOSMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Inhibition of recombinant rat nNOS overexpressed in Escherichia coli using L-arginine as substrate assessed as nitric oxide formation measured for 60...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Inhibition of recombinant rat nNOS overexpressed in Escherichia coli using L-arginine as substrate assessed as nitric oxide formation measured for 60...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:The compounds were evaluated for in vitro inhibition against three NOS isozymes: rat nNOS, bovine eNOS and marine iNOS using known literature methods...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:The compounds were evaluated for in vitro inhibition against three NOS isozymes: rat nNOS, bovine eNOS and marine iNOS using known literature methods...More data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme.More data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Inhibition of nNOS (unknown origin) assessed as conversion of L-[3H]arginine to L-[3H]citrullineMore data for this Ligand-Target Pair
Affinity DataKi: 6.30nMAssay Description:Inhibition against neuronal Nitric Oxide Synthase(nNOS)More data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Inhibition of rat nNOS expressed in Escherichia coli using L-arginine as substrate assessed as formation of nitric oxide by hemoglobin capture assayMore data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Inhibition of rat recombinant nNOS expressed in Escherichia coli using L-arginine as substrate assessed as NO production by hemoglobin capture assayMore data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Inhibition of rat nNOS expressed in Escherichia coli assessed as inhibition of nitric oxide formation by hemoglobin capture assayMore data for this Ligand-Target Pair
Affinity DataKi: 7nMpH: 7.4Assay Description:To test for enzyme inhibition, the hemoglobin capture assay was used to measure nitric oxide production. The assay was performed at 37° C. in HEPES b...More data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Inhibition of recombinant rat nNOS expressed in Escherichia coli using L-arginine as substrate assessed as formation of nitric oxide measured up to 6...More data for this Ligand-Target Pair
Affinity DataKi: 8.70nMAssay Description:Inhibition against neuronal Nitric Oxide Synthase(nNOS)More data for this Ligand-Target Pair
Affinity DataKi: 8.70nMAssay Description:Inhibition of nNOS (unknown origin) assessed as conversion of L-[3H]arginine to L-[3H]citrullineMore data for this Ligand-Target Pair
Affinity DataKi: 8.70nMAssay Description:Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme.More data for this Ligand-Target Pair
Affinity DataKi: 9.70nMAssay Description:Inhibition of rat recombinant nNOS overexpressed in Escherichia coli using L-arginine as substrate assessed as formation of NO-hemoglobin complex mea...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:NOS inhibition assays of representative compounds 1-21 were undertaken, and the results are summarized in Table 1, below. All NOS isoforms were expre...More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibition of recombinant rat nNOS overexpressed in Escherichia coli using L-arginine as substrate assessed as nitric oxide formation measured for 60...More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibition of rat recombinant nNOS expressed in Escherichia coli using L-arginine as substrate assessed as NO production by hemoglobin capture assayMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme.More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme.More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibition of nNOS (unknown origin) assessed as conversion of L-[3H]arginine to L-[3H]citrullineMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibition of nNOS (unknown origin) assessed as conversion of L-[3H]arginine to L-[3H]citrullineMore data for this Ligand-Target Pair
Affinity DataKi: 11nMpH: 7.4Assay Description:To test for enzyme inhibition, the hemoglobin capture assay was used to measure nitric oxide production. The assay was performed at 37° C. in HEPES b...More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:The compounds were evaluated for in vitro inhibition against three NOS isozymes: rat nNOS, bovine eNOS and marine iNOS using known literature methods...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibition of rat neuronal NOS expressed in Escherichia coli using L-arginine as substrate in presence of human oxyhemoglobin after 6 mins by hemoglo...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Enzyme inhibition was evaluated by measuring NO production with the hemoglobin capture assay, which was performed with purified NOSs in 96-well plate...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Enzyme inhibition was evaluated by measuring NO production with the hemoglobin capture assay, which was performed with purified NOSs in 96-well plate...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibition of human neuronal NOS expressed in Escherichia coli using L-arginine as substrate assessed as reduction in NO production by measuring oxid...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibition of human recombinant nNOS expressed in Escherichia coli using L-arginine as substrate assessed as reduction in NO production measured for ...More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibition of rat nNOS using L-arginine substrateMore data for this Ligand-Target Pair
Affinity DataKi: 13.2nMAssay Description:The compounds were evaluated for in vitro inhibition against three NOS isozymes: rat nNOS, bovine eNOS and marine iNOS using known literature methods...More data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Inhibition of rat recombinant nNOS expressed in Escherichia coli using L-arginine as substrate assessed as NO production by hemoglobin capture assayMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Inhibition of rat recombinant nNOS expressed in Escherichia coli by hemoglobin capture assayMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Inhibition of rat recombinant nNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assayMore data for this Ligand-Target Pair
Affinity DataKi: 14nMpH: 7.4Assay Description:To test for enzyme inhibition, the hemoglobin capture assay was used to measure nitric oxide production. The assay was performed at 37° C. in HEPES b...More data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Recombinant NOS isozymes over-expressed in E. coli were utilized. (Ji, H., et al., Discovery of highly potent and selective inhibitors of neuronal ni...More data for this Ligand-Target Pair
Affinity DataKi: 14.7nMAssay Description:The compounds were evaluated for in vitro inhibition against three NOS isozymes: rat nNOS, bovine eNOS and marine iNOS using known literature methods...More data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Inhibitory activity against human neuronal nitric oxide synthase (nNOS) isoenzyme.More data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Inhibition of rat recombinant nNOS expressed in Escherichia coli assessed as nitric oxide formation by hemoglobin capture assayMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Inhibition of rat recombinant nNOS expressed in Escherichia coli assessed as formation of nitric oxide by hemoglobin capture assayMore data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)