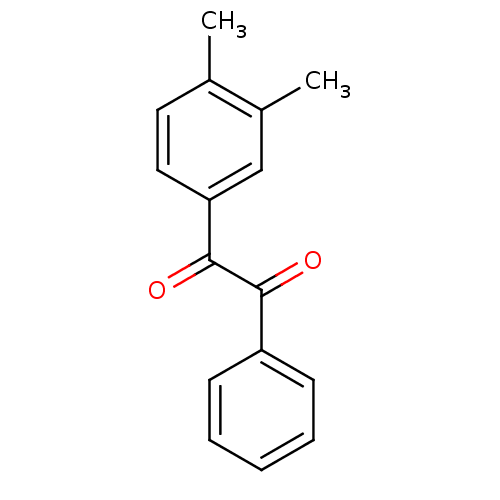
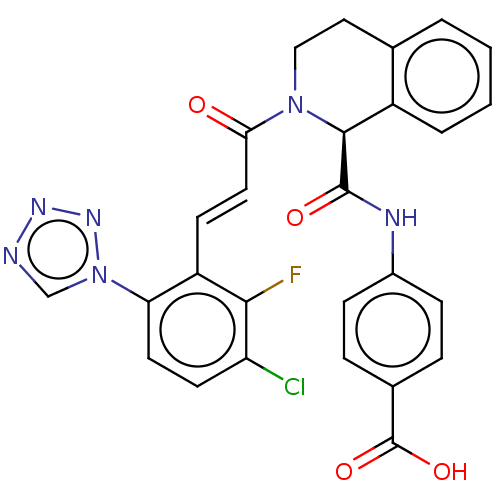
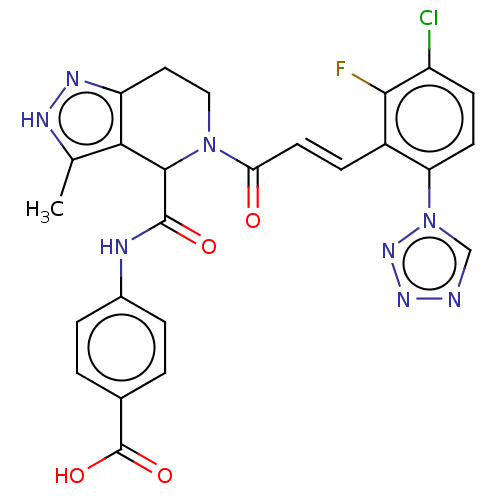
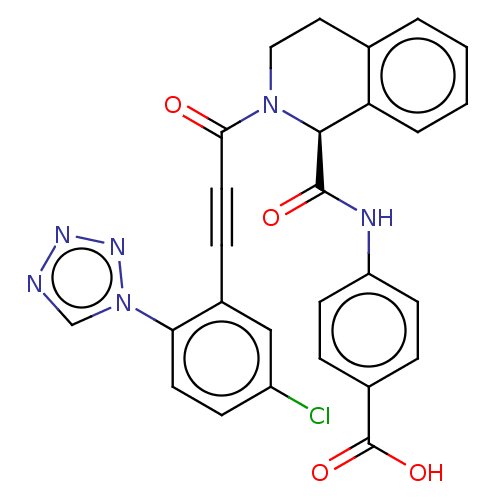
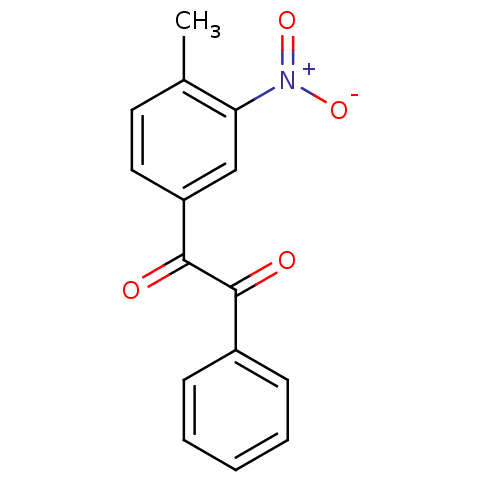
Affinity DataKi: 0.100nM ΔG°: -59.4kJ/molepH: 7.4 T: 2°CAssay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
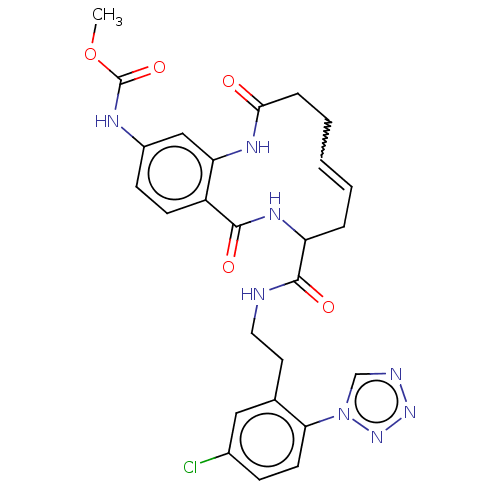
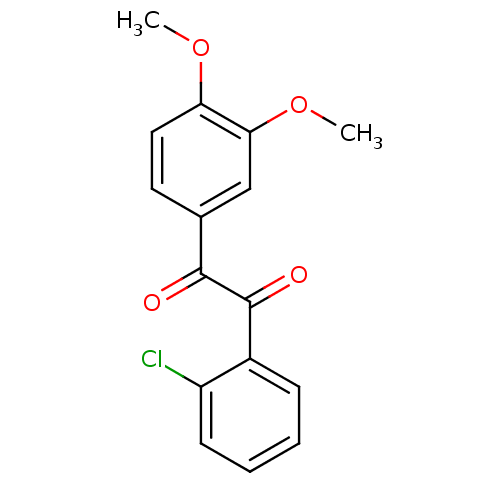
Affinity DataKi: 0.100nM ΔG°: -59.4kJ/molepH: 7.4 T: 2°CAssay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
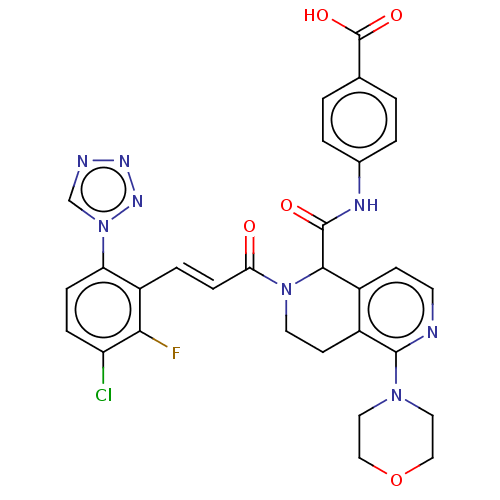
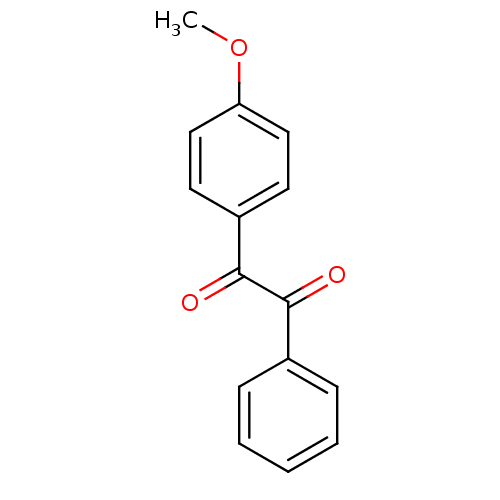
Affinity DataKi: 0.100nM ΔG°: -59.4kJ/molepH: 7.4 T: 2°CAssay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
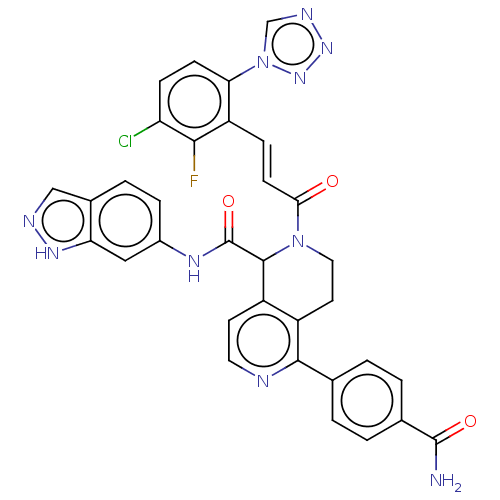
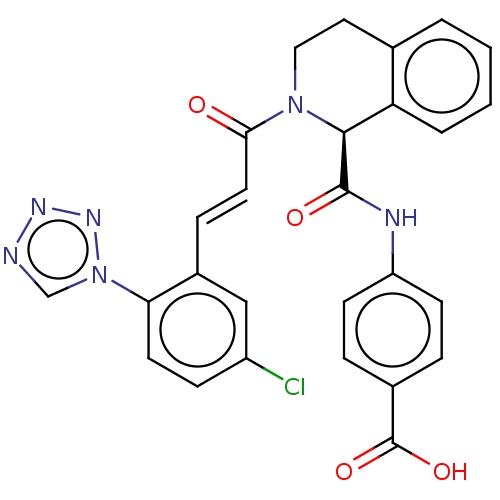
Affinity DataKi: 0.100nM ΔG°: -59.4kJ/molepH: 7.4 T: 2°CAssay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
Affinity DataKi: 0.100nM ΔG°: -59.4kJ/molepH: 7.4 T: 2°CAssay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
Affinity DataKi: 0.100nM ΔG°: -59.4kJ/molepH: 7.4 T: 2°CAssay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nM ΔG°: -57.6kJ/molepH: 7.4 T: 2°CAssay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nM ΔG°: -57.6kJ/molepH: 7.4 T: 2°CAssay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nM ΔG°: -57.6kJ/molepH: 7.4 T: 2°CAssay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nM ΔG°: -57.6kJ/molepH: 7.4 T: 2°CAssay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nM ΔG°: -54.8kJ/molepH: 7.4 T: 2°CAssay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nM ΔG°: -52.6kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.700nM ΔG°: -52.3kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 0.720nM ΔG°: -52.2kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 2.30nM ΔG°: -49.3kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 2.52nM ΔG°: -49.1kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 2.82nM ΔG°: -48.8kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 3.08nMpH: 7.4Assay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba...More data for this Ligand-Target Pair
Affinity DataKi: 3.22nM ΔG°: -48.5kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 4.10nM ΔG°: -47.9kJ/molepH: 7.4 T: 2°CAssay Description:CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w...More data for this Ligand-Target Pair
Affinity DataKi: 4.21nM ΔG°: -47.8kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: <5nMpH: 7.4Assay Description:The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm...More data for this Ligand-Target Pair
Affinity DataKi: 5nM ΔG°: -47.4kJ/molepH: 7.4 T: 2°CAssay Description:CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w...More data for this Ligand-Target Pair
Affinity DataKi: <5nMpH: 7.4Assay Description:The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm...More data for this Ligand-Target Pair
Affinity DataKi: <5nMpH: 7.4Assay Description:The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm...More data for this Ligand-Target Pair
Affinity DataKi: <5nMpH: 7.4Assay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
Affinity DataKi: <5nMpH: 7.4Assay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
Affinity DataKi: <5nMpH: 7.4Assay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
Affinity DataKi: <5nMpH: 7.4Assay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
Affinity DataKi: <5nMpH: 7.4Assay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
Affinity DataKi: <5nMpH: 7.4Assay Description:The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm...More data for this Ligand-Target Pair
Affinity DataKi: <5nMpH: 7.4Assay Description:The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm...More data for this Ligand-Target Pair
Affinity DataKi: <5nMpH: 7.4Assay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
Affinity DataKi: 5.02nMpH: 7.4Assay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
Affinity DataKi: 5.60nM ΔG°: -47.1kJ/molepH: 7.4 T: 2°CAssay Description:CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w...More data for this Ligand-Target Pair
Affinity DataKi: 5.68nMpH: 7.4Assay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
Affinity DataKi: 6.24nMpH: 7.4Assay Description:The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm...More data for this Ligand-Target Pair
Affinity DataKi: 7.08nMpH: 7.4Assay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
Affinity DataKi: 7.51nM ΔG°: -46.4kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 7.70nM ΔG°: -46.3kJ/molepH: 7.4 T: 2°CAssay Description:CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w...More data for this Ligand-Target Pair
Affinity DataKi: 7.90nM ΔG°: -46.2kJ/molepH: 7.4 T: 2°CAssay Description:CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w...More data for this Ligand-Target Pair
Affinity DataKi: 8.16nMpH: 7.4Assay Description:The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm...More data for this Ligand-Target Pair
Affinity DataKi: 8.90nM ΔG°: -46.0kJ/molepH: 7.4 T: 2°CAssay Description:CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w...More data for this Ligand-Target Pair
Affinity DataKi: 9nM ΔG°: -45.9kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 9.5nM ΔG°: -45.8kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 10.2nM ΔG°: -45.6kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 10.3nM ΔG°: -45.6kJ/molepH: 7.4 T: 2°CAssay Description:CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w...More data for this Ligand-Target Pair
Affinity DataKi: 10.3nMpH: 7.4Assay Description:Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 145 mM NaCl, 5 mM KCl, and 0.1% PEG 8000 (polyethylene glycol; JT Bake...More data for this Ligand-Target Pair
Affinity DataKi: 12nM ΔG°: -45.2kJ/molepH: 7.4 T: 2°CAssay Description:The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine...More data for this Ligand-Target Pair
Affinity DataKi: 14.1nMpH: 7.4Assay Description:The effectiveness of compounds of the present invention as inhibitors of the coagulation Factors XIa, VIIa, IXa, Xa, XIIa, plasma kallikrein or throm...More data for this Ligand-Target Pair


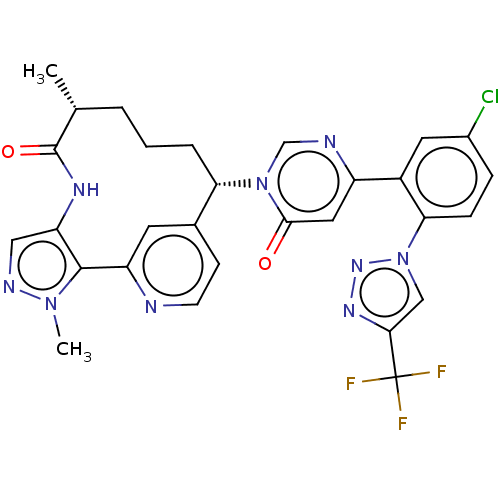


 3D Structure (crystal)
3D Structure (crystal)