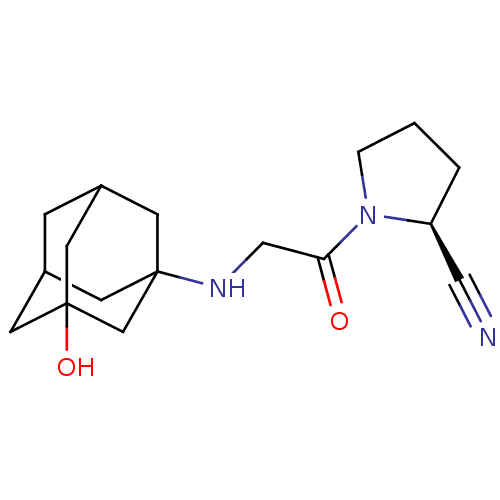
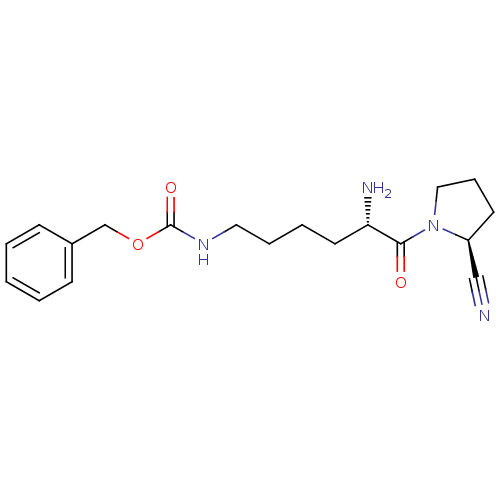
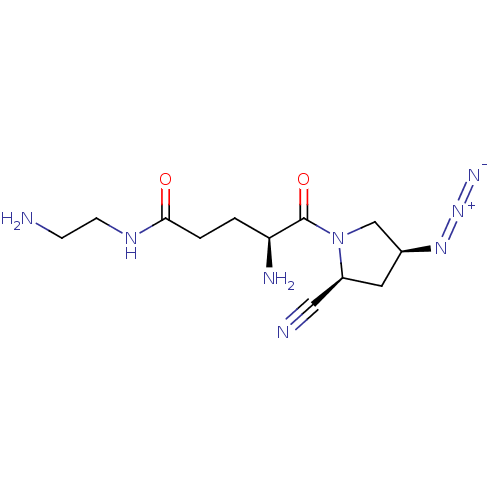
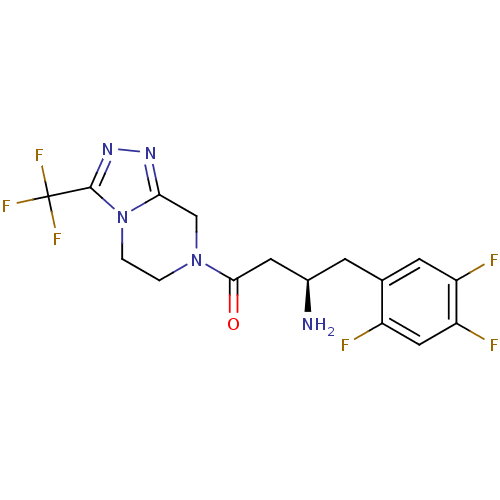
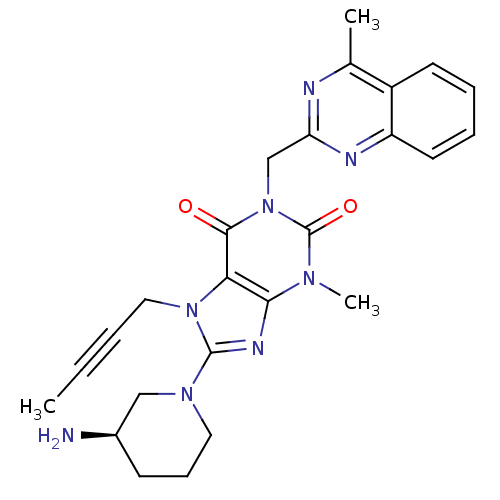
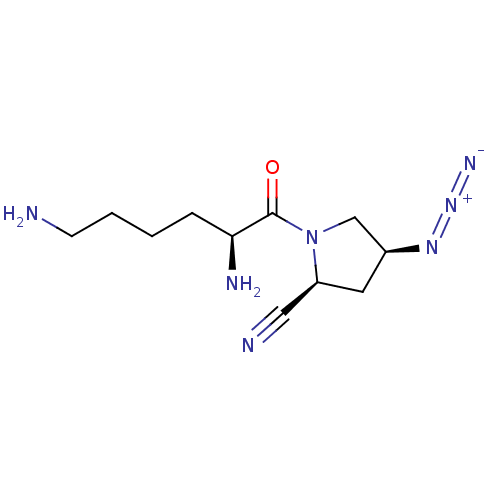
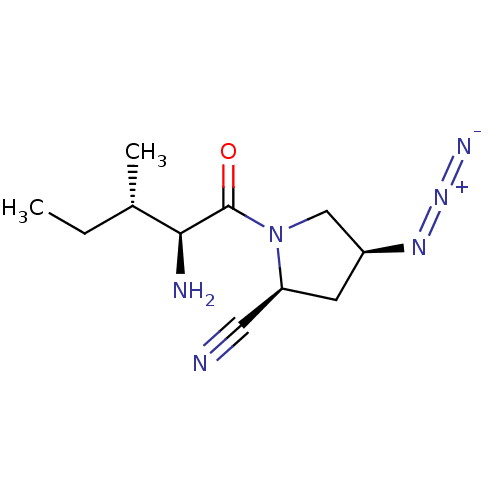
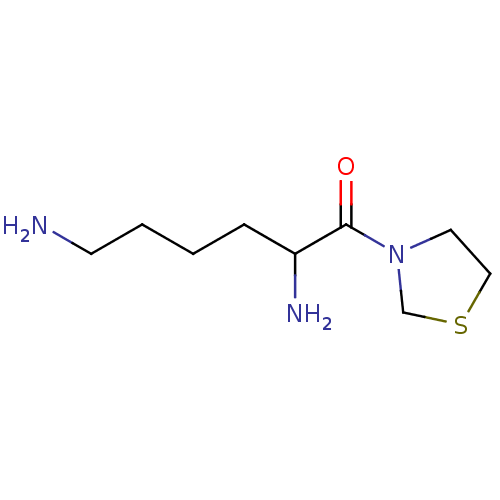
Affinity DataKi: 17nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrate preincubate...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 79nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.50E+3nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 140nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 330nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 310nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.10E+3nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 9.40E+3nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 580nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of Porphyromonas gingivalis N-terminal His-tagged DPP4 expressed in Escherichia coli using Gly-Pro-p-nitroanilide as substrateMore data for this Ligand-Target Pair