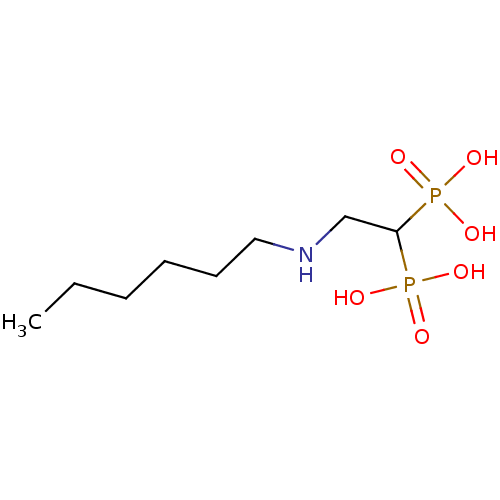
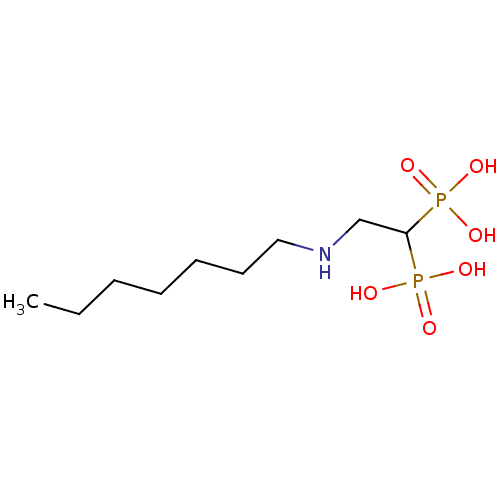
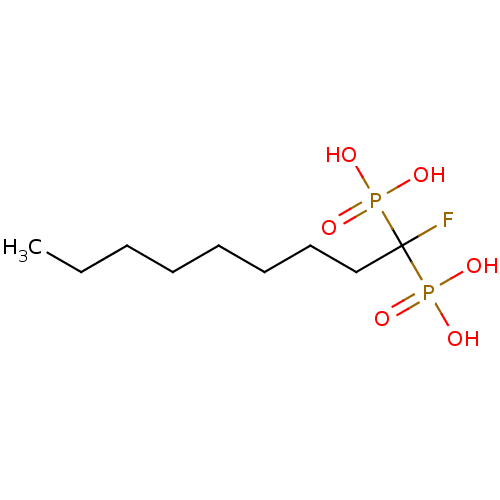
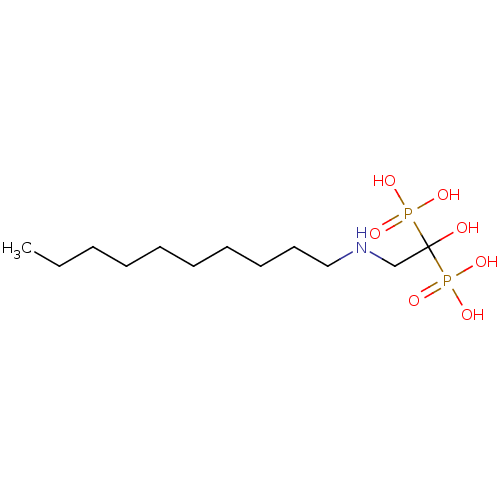
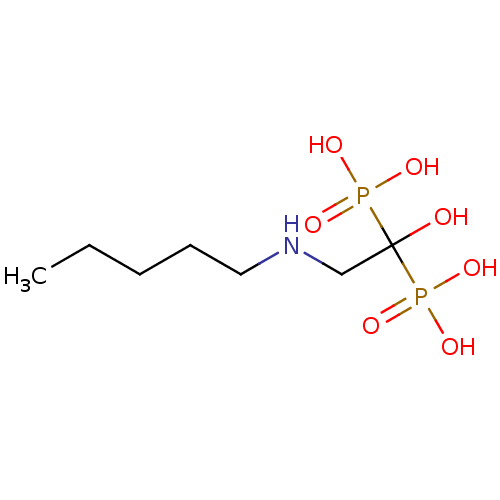
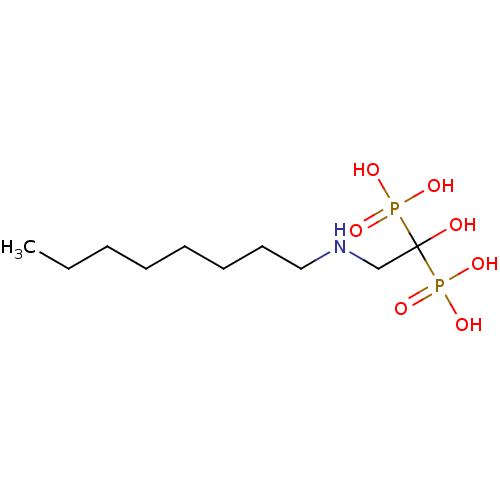
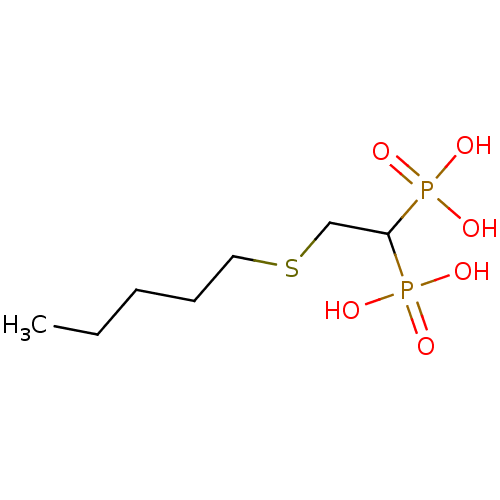
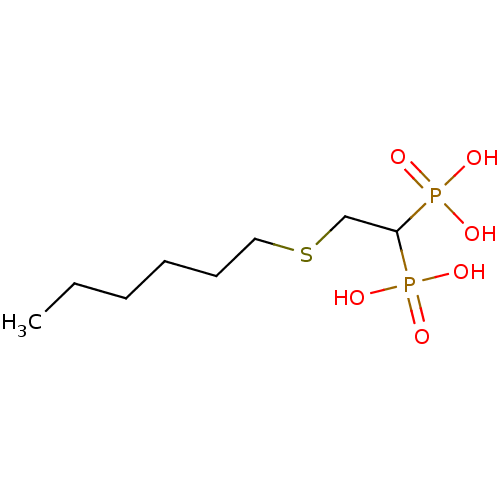
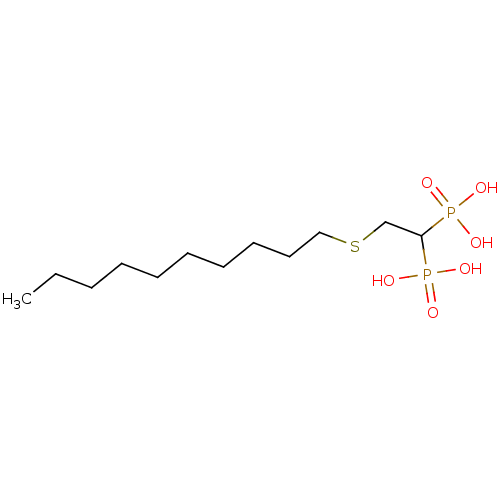
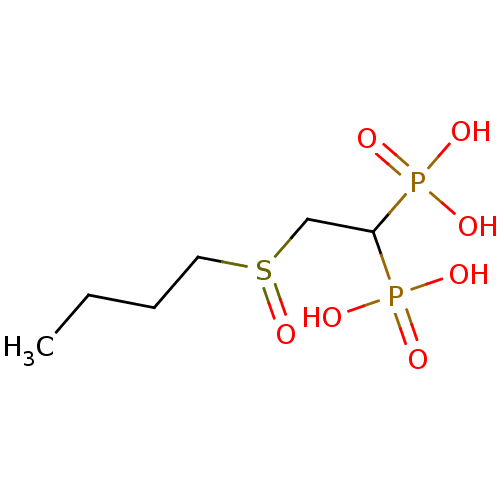
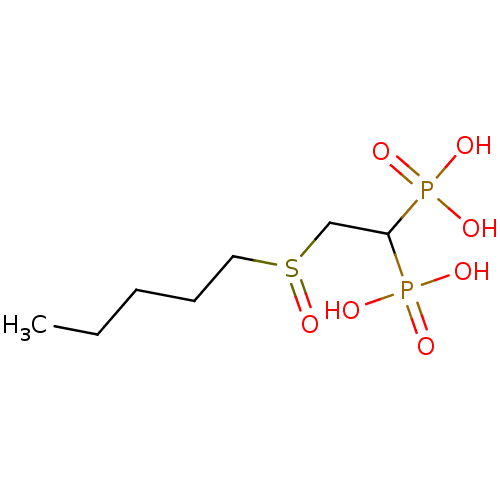
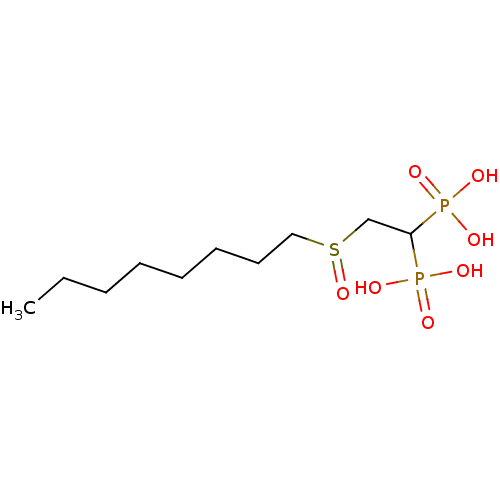
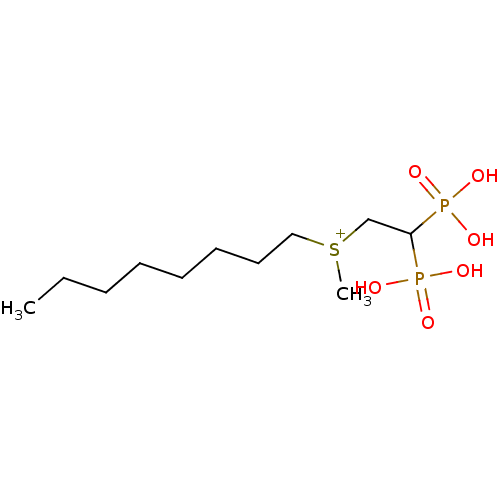
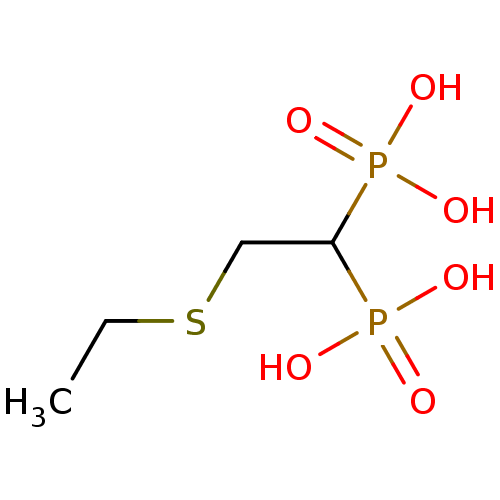
Affinity DataKi: 400nMAssay Description:Inhibition of Trypanosoma cruzi FPPSMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Inhibition of Trypanosoma cruzi FPPS after 30 mins using [14C]IPP by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 430nMAssay Description:Inhibition of Trypanosoma cruzi FPPS after 30 mins using [14C]IPP by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Inhibition of Trypanosoma cruzi FPPS after 30 mins using [14C]IPP by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 856nMAssay Description:Inhibition of Trypanosoma cruzi FPPS after 30 mins using [14C]IPP by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 811nMAssay Description:Inhibition of Trypanosoma cruzi FPPS after 30 mins using [14C]IPP by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Inhibition of Trypanosoma cruzi FPPS after 30 mins using [14C]IPP by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Inhibition of Trypanosoma cruzi FPPS after 30 mins using [14C]IPP by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.01E+3nMAssay Description:Inhibition of Trypanosoma cruzi FPPS after 30 mins using [14C]IPP by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 490nMAssay Description:Inhibition of Trypanosoma cruzi FPPS after 30 mins using [14C]IPP by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 58nMAssay Description:Inhibition of Trypanosoma cruzi FPPS after 30 mins using [14C]IPP by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 440nMAssay Description:Inhibition of Toxoplasma gondii FPPS after 30 mins using [14C]IPP by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 68nMAssay Description:Inhibition of Toxoplasma gondii FPPS after 30 mins using [14C]IPP by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 868nMAssay Description:Inhibition of Toxoplasma gondii FPPS after 30 mins using [14C]IPP by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 93nMAssay Description:Inhibition of Toxoplasma gondii FPPS after 30 mins using [14C]IPP by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 292nMAssay Description:Inhibition of Toxoplasma gondii FPPS after 30 mins using [14C]IPP by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 74nMAssay Description:Inhibition of Toxoplasma gondii FPPS after 30 mins using [14C]IPP by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 140nMAssay Description:Inhibition of Toxoplasma gondii FPPS after 30 mins using [14C]IPP by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 35nMAssay Description:Inhibition of Toxoplasma gondii FPPSMore data for this Ligand-Target Pair
Affinity DataIC50: 125nMAssay Description:Inhibition of Toxoplasma gondii FPPSMore data for this Ligand-Target Pair
Affinity DataIC50: 95nMAssay Description:Inhibition of Toxoplasma gondii FPPSMore data for this Ligand-Target Pair
Affinity DataIC50: 74nMAssay Description:Inhibition of Toxoplasma gondii recombinant FPPS expressed in Escherichia coli using [4-14C]IPP/DMAPP/GPP as substrate after 30 mins by scintillation...More data for this Ligand-Target Pair
Affinity DataIC50: 67nMAssay Description:Inhibition of Toxoplasma gondii FPPSMore data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:Inhibition of Toxoplasma gondii FPPSMore data for this Ligand-Target Pair
Affinity DataIC50: 39nMAssay Description:Inhibition of Toxoplasma gondii FPPSMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Inhibition of Toxoplasma gondii FPPSMore data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of Toxoplasma gondii FPPSMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Trypanosoma cruzi farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Trypanosoma cruzi farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Trypanosoma cruzi farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: 6.39E+3nMAssay Description:Inhibition of Trypanosoma cruzi farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of Trypanosoma cruzi farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: 97nMAssay Description:Inhibition of Trypanosoma cruzi farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Trypanosoma cruzi farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Trypanosoma cruzi farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Trypanosoma cruzi farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Trypanosoma cruzi farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Trypanosoma cruzi farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Trypanosoma cruzi farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Trypanosoma cruzi farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Trypanosoma cruzi farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of Trypanosoma cruzi farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Inhibition of Trypanosoma cruzi farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Toxoplasma gondii farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Toxoplasma gondii farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Toxoplasma gondii farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inhibition of Toxoplasma gondii farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: 25nMAssay Description:Inhibition of Toxoplasma gondii farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Inhibition of Toxoplasma gondii farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair
Affinity DataIC50: 1.83E+3nMAssay Description:Inhibition of Toxoplasma gondii farnesyl diphosphate synthase assessed as incorporation of [4-14C]IPP measured at 37 degCMore data for this Ligand-Target Pair


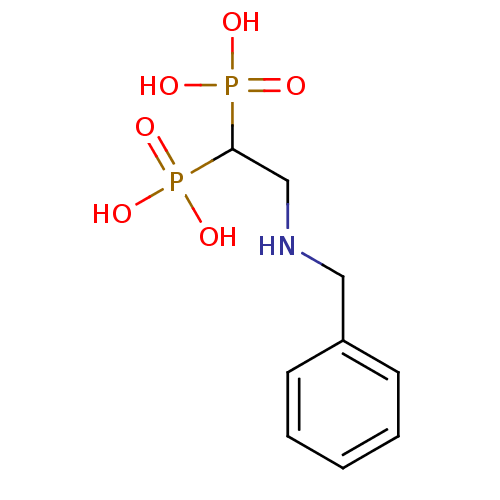
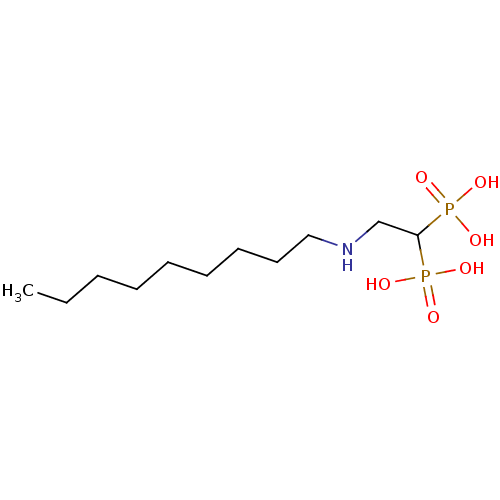
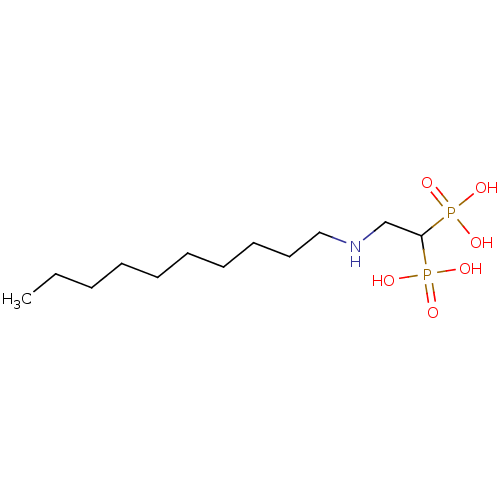
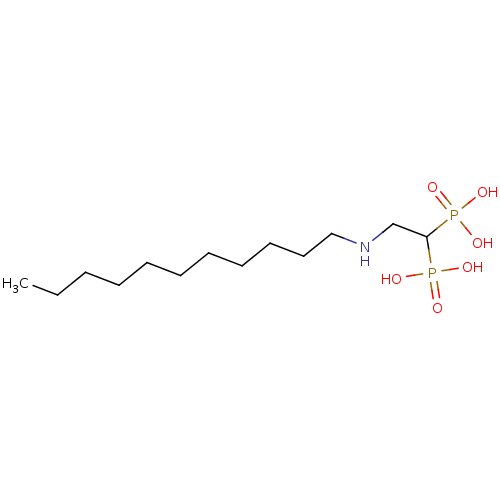
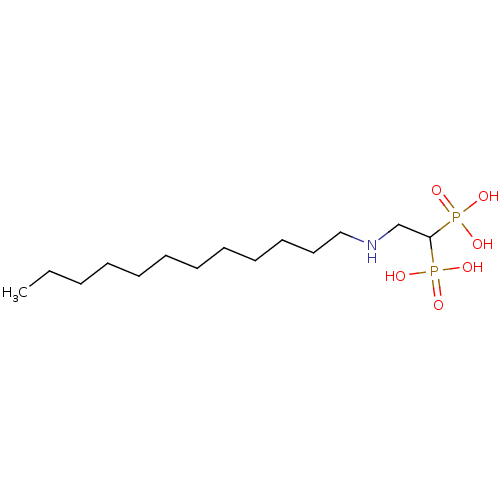
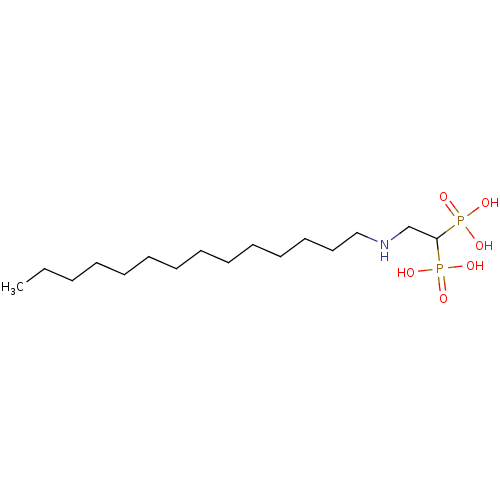
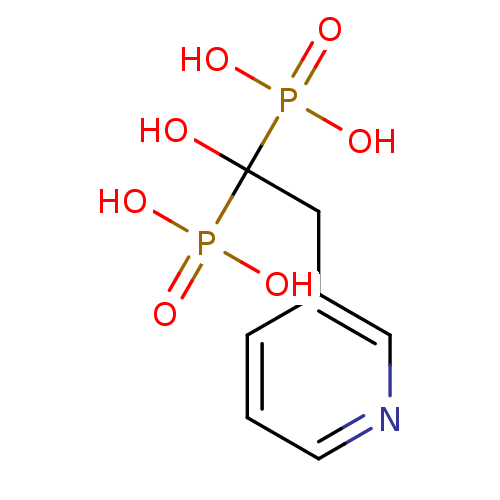
 3D Structure (crystal)
3D Structure (crystal)