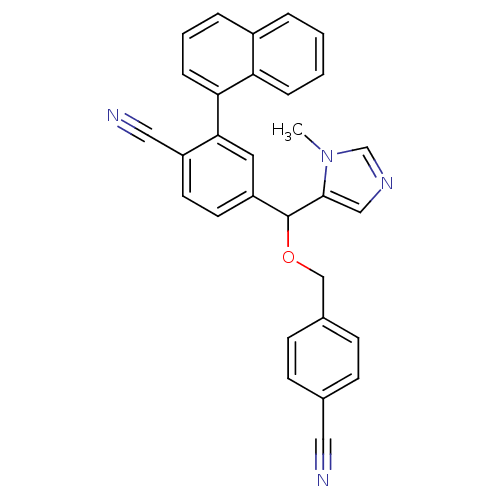
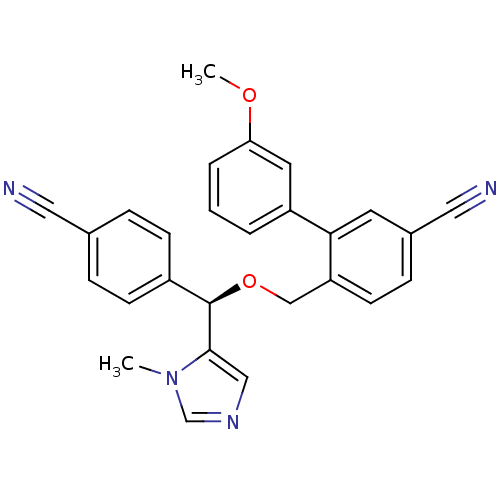
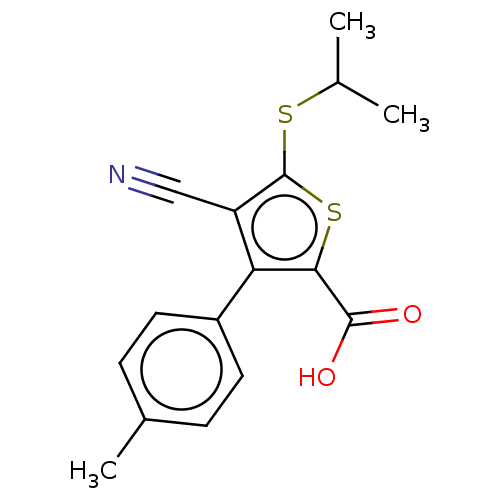
TargetProtein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Globe Pharmaceutical R And Abbott Laboratories
Globe Pharmaceutical R And Abbott Laboratories
Affinity DataIC50: 8.90nMpH: 7.0 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we...More data for this Ligand-Target Pair
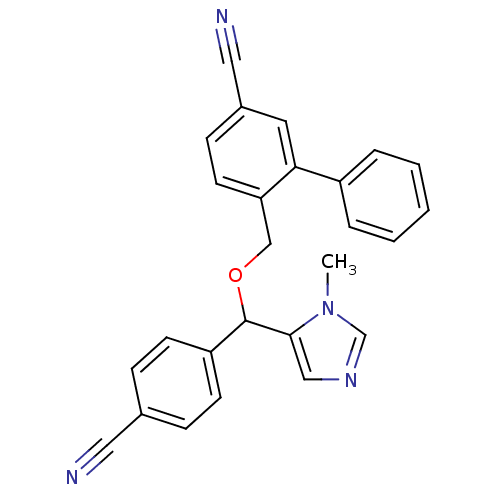
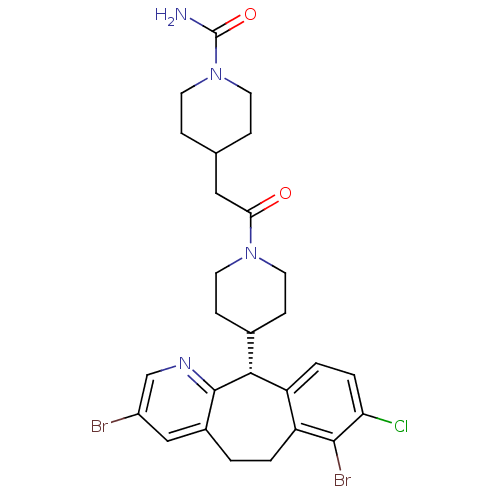
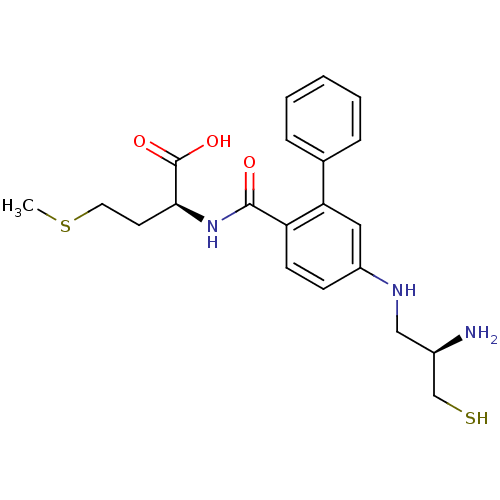
TargetProtein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Globe Pharmaceutical R And Abbott Laboratories
Globe Pharmaceutical R And Abbott Laboratories
Affinity DataIC50: 0.300nMpH: 7.0 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we...More data for this Ligand-Target Pair
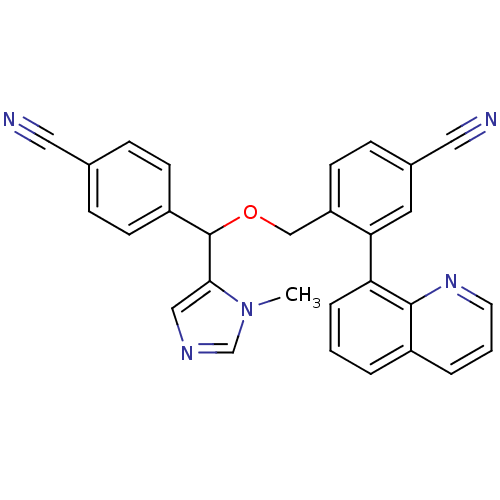
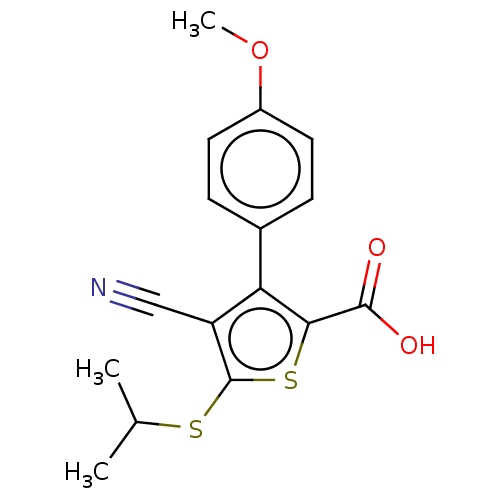
TargetProtein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Globe Pharmaceutical R And Abbott Laboratories
Globe Pharmaceutical R And Abbott Laboratories
Affinity DataIC50: 4.80nMpH: 7.0 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we...More data for this Ligand-Target Pair
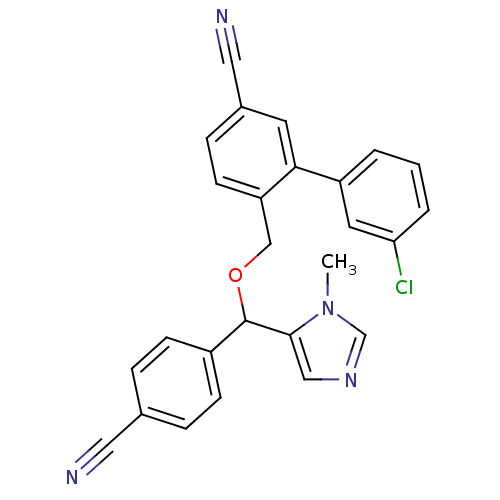
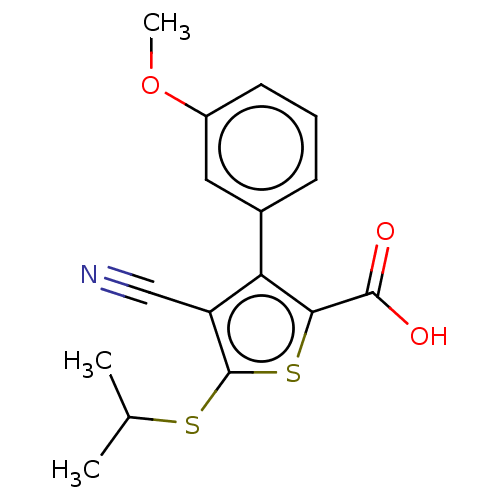
TargetProtein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Globe Pharmaceutical R And Abbott Laboratories
Globe Pharmaceutical R And Abbott Laboratories
Affinity DataIC50: 0.910nMpH: 7.0 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Globe Pharmaceutical R And Abbott Laboratories
Globe Pharmaceutical R And Abbott Laboratories
Affinity DataIC50: 1.10nMpH: 7.0 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Globe Pharmaceutical R And Abbott Laboratories
Globe Pharmaceutical R And Abbott Laboratories
Affinity DataIC50: 1.30nMpH: 7.0 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Globe Pharmaceutical R And Abbott Laboratories
Globe Pharmaceutical R And Abbott Laboratories
Affinity DataIC50: 0.940nMpH: 7.0 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Globe Pharmaceutical R And Abbott Laboratories
Globe Pharmaceutical R And Abbott Laboratories
Affinity DataIC50: 5.20nMpH: 7.0 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Globe Pharmaceutical R And Abbott Laboratories
Globe Pharmaceutical R And Abbott Laboratories
Affinity DataIC50: 0.870nMpH: 7.0 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Globe Pharmaceutical R And Abbott Laboratories
Globe Pharmaceutical R And Abbott Laboratories
Affinity DataIC50: 0.75nMpH: 7.0 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Globe Pharmaceutical R And Abbott Laboratories
Globe Pharmaceutical R And Abbott Laboratories
Affinity DataIC50: 0.350nMpH: 7.0 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Globe Pharmaceutical R And Abbott Laboratories
Globe Pharmaceutical R And Abbott Laboratories
Affinity DataIC50: 21nMpH: 7.0 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Globe Pharmaceutical R And Abbott Laboratories
Globe Pharmaceutical R And Abbott Laboratories
Affinity DataIC50: 0.870nMpH: 7.0 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Globe Pharmaceutical R And Abbott Laboratories
Globe Pharmaceutical R And Abbott Laboratories
Affinity DataIC50: 1.10nMpH: 7.0 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Globe Pharmaceutical R And Abbott Laboratories
Globe Pharmaceutical R And Abbott Laboratories
Affinity DataIC50: 0.690nMpH: 7.0 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Globe Pharmaceutical R And Abbott Laboratories
Globe Pharmaceutical R And Abbott Laboratories
Affinity DataIC50: 0.800nMpH: 7.0 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataIC50: 62nMpH: 7.5 T: 2°CAssay Description:FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataIC50: 5nMpH: 7.5 T: 2°CAssay Description:FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataIC50: 78nMpH: 7.5 T: 2°CAssay Description:FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataIC50: 2.30nMpH: 7.5 T: 2°CAssay Description:FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataIC50: >99nMpH: 7.5 T: 2°CAssay Description:FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataIC50: 6nMpH: 7.5 T: 2°CAssay Description:FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataIC50: 86nMpH: 7.5 T: 2°CAssay Description:FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataIC50: 3.10nMpH: 7.5 T: 2°CAssay Description:FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Homo sapiens (Human))
Schering-Plough Research Institute
Schering-Plough Research Institute
Affinity DataIC50: >280nMpH: 7.5 T: 2°CAssay Description:FPT activity was determined by measuring transfer of [3H] farnesyl from [3H]farnesyl pyrophosphate to the substrate His6-Ha-Ras-CVLS. The incorporate...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 110nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 1.30E+3nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 1.40E+3nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 270nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 460nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 1.30E+3nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 580nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 1.60E+3nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 250nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 1.60E+3nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 9.70E+3nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 85nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 620nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 610nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 190nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 1.40E+3nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 3.10E+3nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 1.10E+3nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 320nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 2.70E+3nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 640nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 350nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 1.00E+3nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Saccharomyces cerevisiae (Baker's yeast))
Centre De Recherche De Gif
Centre De Recherche De Gif
Affinity DataIC50: 0.780nMpH: 7.5Assay Description:Assays were realized on 96-well plates, prepared with Biomek NKMC and Biomek 3000 from Beckman Coulter and read on Wallac Victor fluorimeter from Per...More data for this Ligand-Target Pair
TargetProtein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha(Bos taurus (bovine))
Abbott Laboratories
Abbott Laboratories
Affinity DataIC50: 1.40nMpH: 7.5 T: 2°CAssay Description:The in vitro activity of compounds inhibiting FTase was determined by measuring the transfer of [3H]-FPP to substrate Ha-Ras-CVLS. The incorporated r...More data for this Ligand-Target Pair