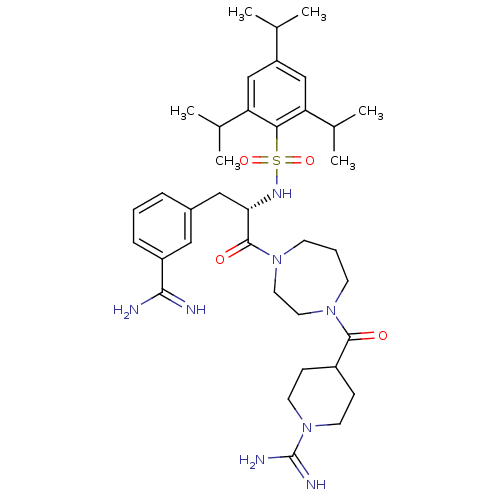
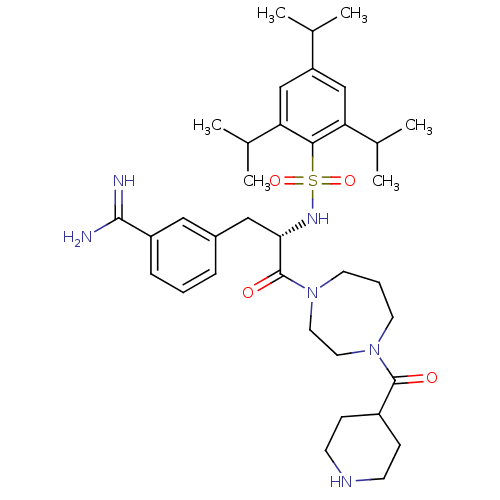
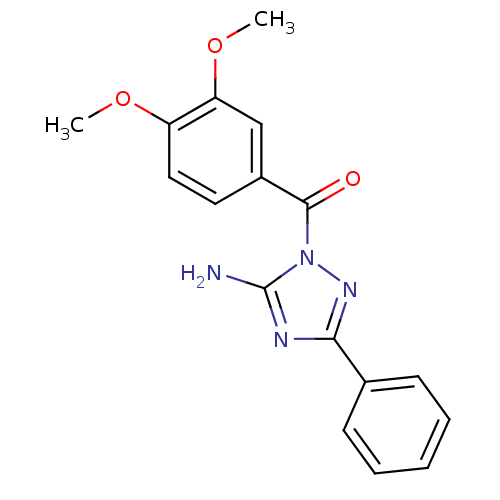
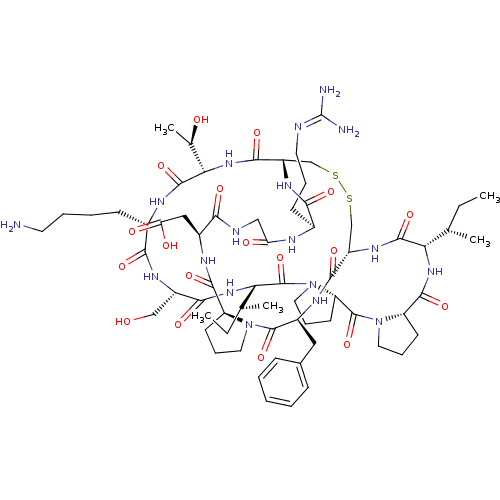
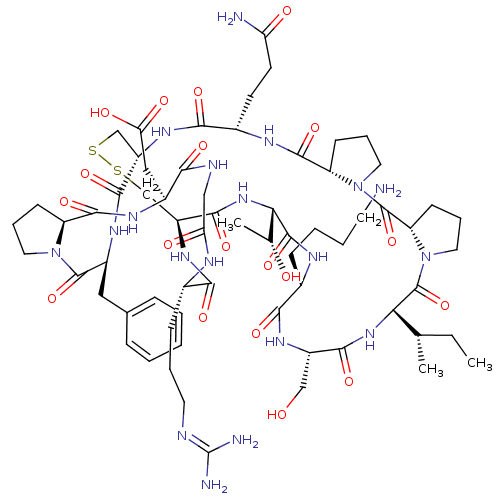
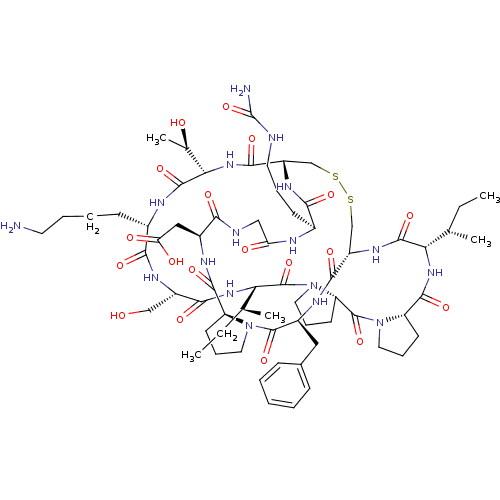
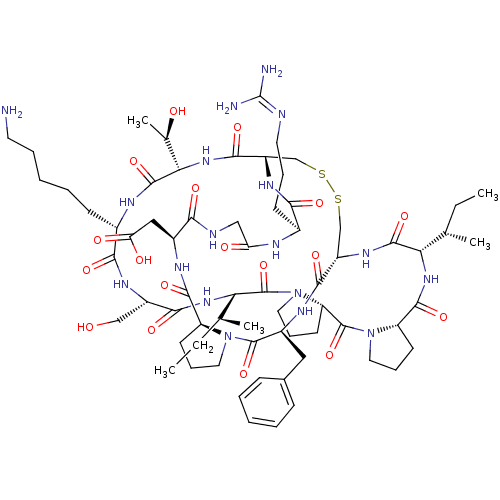
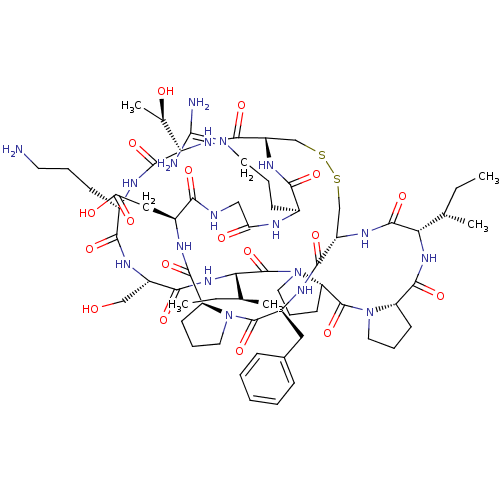
Affinity DataKi: 0.0110nM ΔG°: -62.5kJ/molepH: 7.4 T: 2°CAssay Description:Enzymatic assays were performed in the following reaction buffer: 50 mM HEPES, pH 7.4 containing 500 μg/ml bovine serum albumin. Enzyme activiti...More data for this Ligand-Target Pair
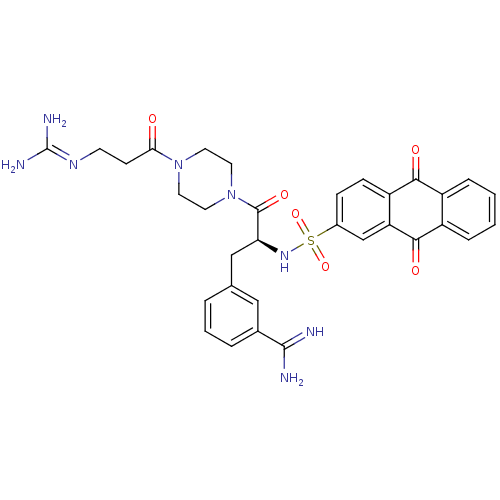
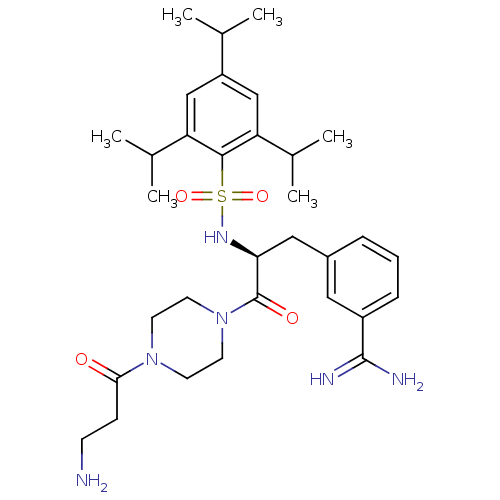
Affinity DataKi: 0.0880nM ΔG°: -57.4kJ/molepH: 7.4 T: 2°CAssay Description:Enzymatic assays were performed in the following reaction buffer: 50 mM HEPES, pH 7.4 containing 500 μg/ml bovine serum albumin. Enzyme activiti...More data for this Ligand-Target Pair
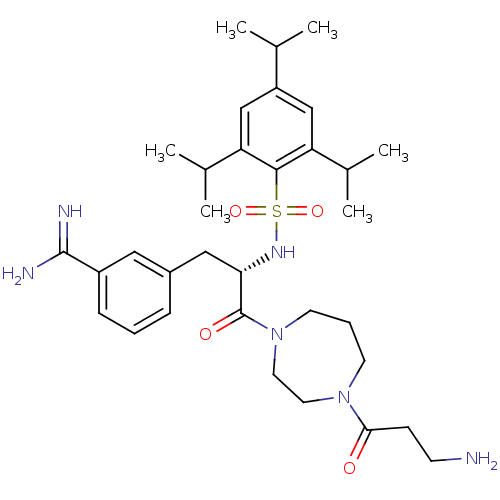
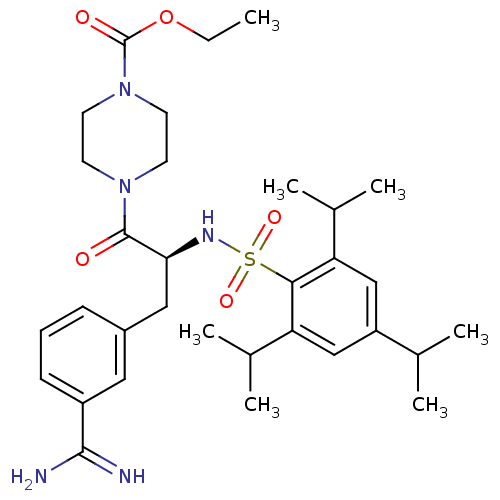
Affinity DataKi: 1.40nM ΔG°: -50.5kJ/molepH: 7.4 T: 2°CAssay Description:Enzymatic assays were performed in the following reaction buffer: 50 mM HEPES, pH 7.4 containing 500 μg/ml bovine serum albumin. Enzyme activiti...More data for this Ligand-Target Pair
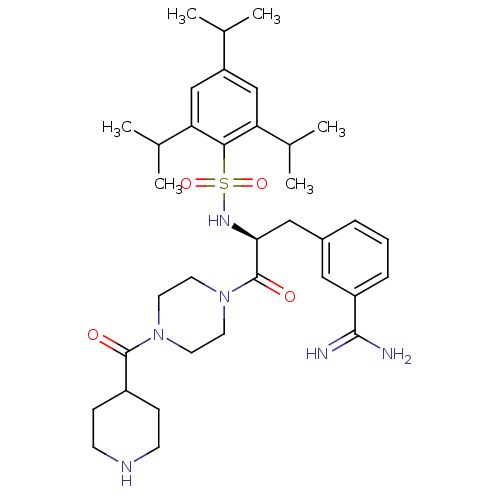
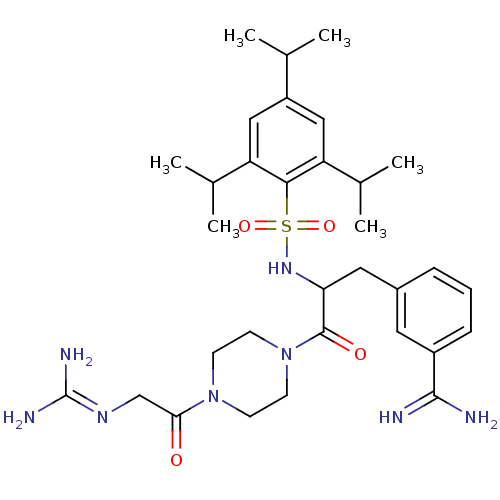
Affinity DataKi: 4.60nM ΔG°: -47.6kJ/molepH: 7.4 T: 2°CAssay Description:Enzymatic assays were performed in the following reaction buffer: 50 mM HEPES, pH 7.4 containing 500 μg/ml bovine serum albumin. Enzyme activiti...More data for this Ligand-Target Pair
Affinity DataKi: 9.5nM ΔG°: -45.8kJ/molepH: 7.4 T: 2°CAssay Description:Enzymatic assays were performed in the following reaction buffer: 50 mM HEPES, pH 7.4 containing 500 μg/ml bovine serum albumin. Enzyme activiti...More data for this Ligand-Target Pair
Affinity DataKi: 457nM ΔG°: -36.2kJ/molepH: 7.4 T: 2°CAssay Description:Enzymatic assays were performed in the following reaction buffer: 50 mM HEPES, pH 7.4 containing 500 μg/ml bovine serum albumin. Enzyme activiti...More data for this Ligand-Target Pair
Affinity DataKi: 6.12E+3nM ΔG°: -29.8kJ/molepH: 7.4 T: 2°CAssay Description:Enzymatic assays were performed in the following reaction buffer: 50 mM HEPES, pH 7.4 containing 500 μg/ml bovine serum albumin. Enzyme activiti...More data for this Ligand-Target Pair
Affinity DataKi: 14nM ΔG°: -44.8kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 44nM ΔG°: -42.0kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 50nM ΔG°: -41.7kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 53nM ΔG°: -41.5kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 57nM ΔG°: -41.3kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 61nM ΔG°: -41.2kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 61nM ΔG°: -41.2kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 65nM ΔG°: -41.0kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 89nM ΔG°: -40.2kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 96nM ΔG°: -40.1kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 100nM ΔG°: -40.0kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 130nM ΔG°: -39.3kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 140nM ΔG°: -39.1kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 170nM ΔG°: -38.6kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 200nM ΔG°: -38.2kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 210nM ΔG°: -38.1kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 250nM ΔG°: -37.7kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 320nM ΔG°: -37.1kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 330nM ΔG°: -37.0kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 340nM ΔG°: -36.9kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 400nM ΔG°: -36.5kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 690nM ΔG°: -35.2kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 690nM ΔG°: -35.2kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 730nM ΔG°: -35.0kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataKi: 1.50E+3nM ΔG°: -33.2kJ/molepH: 8.0 T: 2°CAssay Description:The measurements were carried out on a microplate reader. Two concentrations of the substrate and five concentrations of the inhibitor were used. Af...More data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMpH: 8.0Assay Description:Inhibition of human matriptase measured after 15 mins at pH 8 by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMpH: 8.0Assay Description:Inhibition of human matriptase measured after 15 mins at pH 8 by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMpH: 8.0Assay Description:Inhibition of human matriptase measured after 15 mins at pH 8 by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 100nM ΔG°: -39.6kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 160nM ΔG°: -38.4kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 380nM ΔG°: -36.3kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 450nM ΔG°: -35.9kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 857nM ΔG°: -34.3kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 890nM ΔG°: -34.2kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nM ΔG°: -33.9kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 2.33E+3nM ΔG°: -31.8kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 4.75E+3nM ΔG°: -30.1kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 2.25E+4nM ΔG°: -26.3kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 2.80E+4nM ΔG°: -25.7kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 9.00E+4nM ΔG°: -22.9kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 1.07E+5nM ΔG°: -22.4kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: >2.50E+5nM ΔG°: >-20.4kJ/molepH: 8.5 T: 2°CAssay Description:The enzyme reaction was initiated by adding fluorescence peptide substrate to reaction mixture containing enzyme and test compounds. The enzyme activ...More data for this Ligand-Target Pair
Affinity DataKi: 0.0110nMAssay Description:Tight binding inhibition of human matriptase expressed in Drosophila melanogaster S2 cells using Boc-QAR-AMC as substrate incubated for 15 mins prior...More data for this Ligand-Target Pair


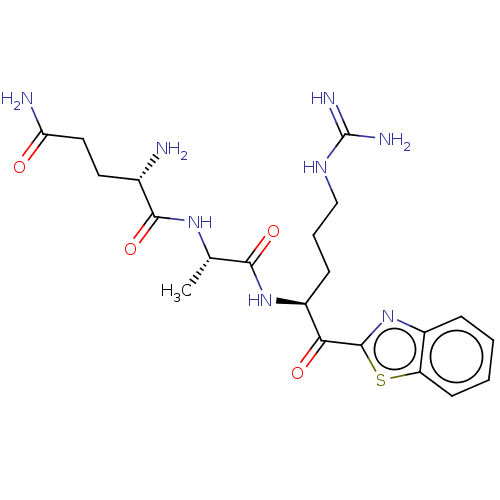

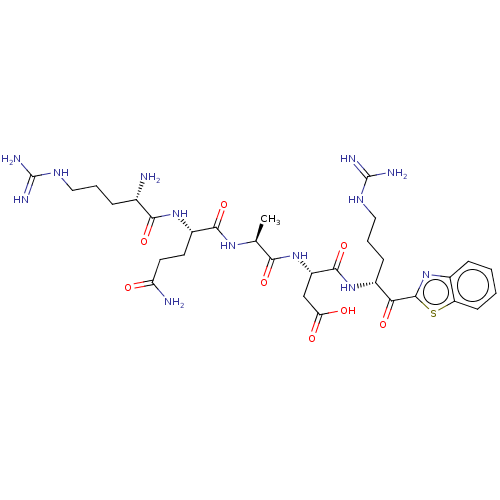



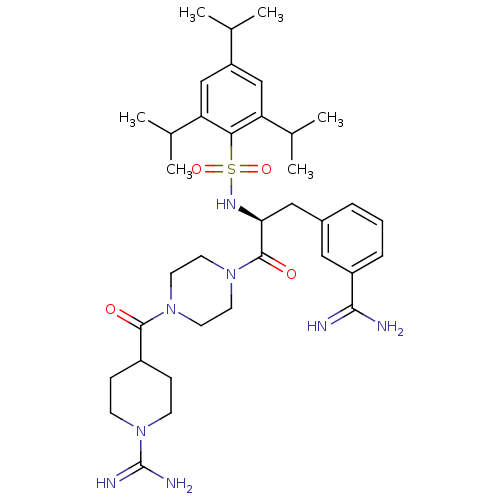
 3D Structure (crystal)
3D Structure (crystal)