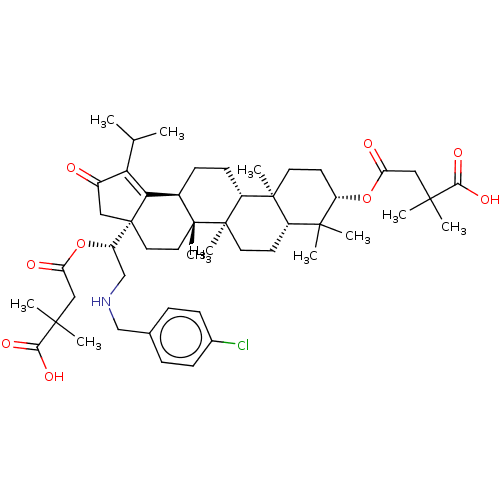
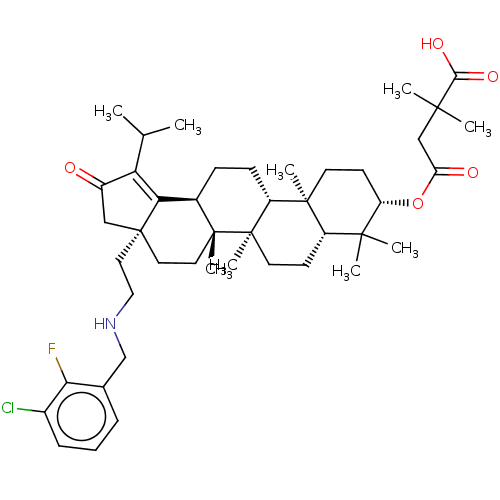
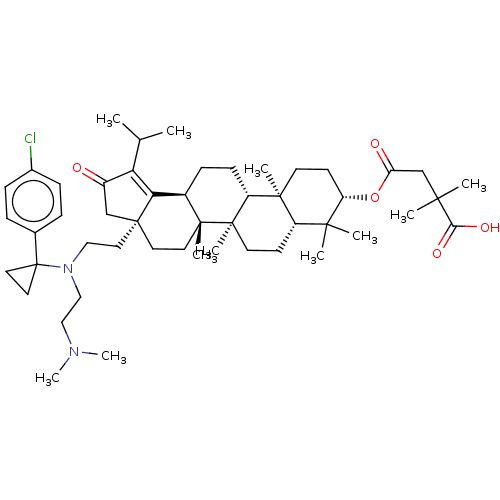
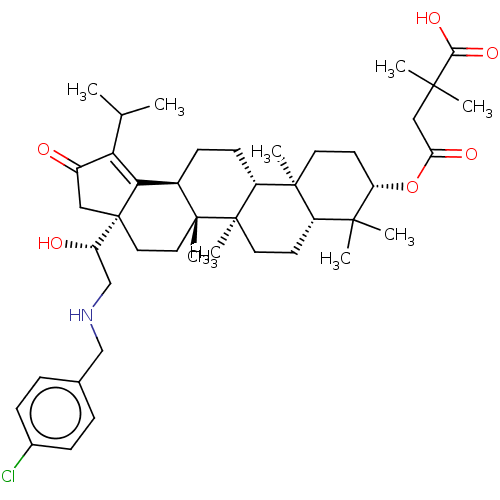
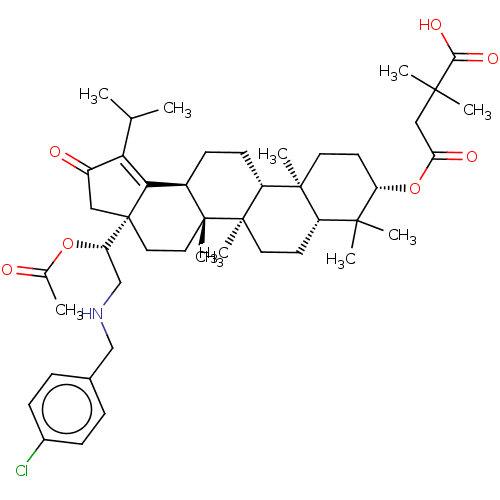
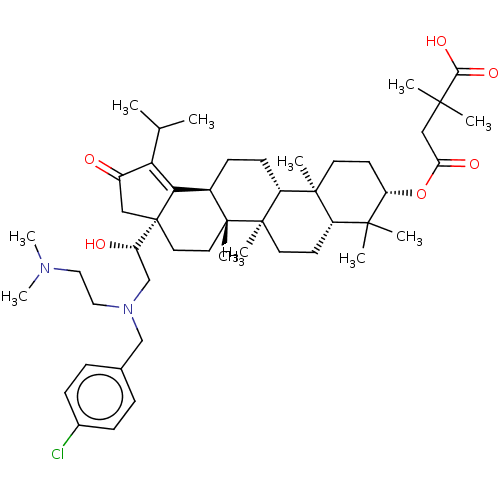
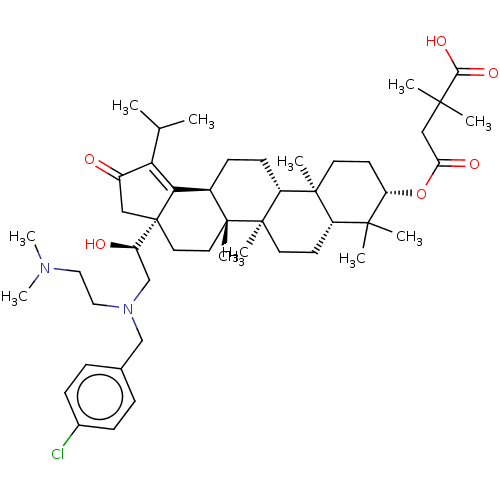
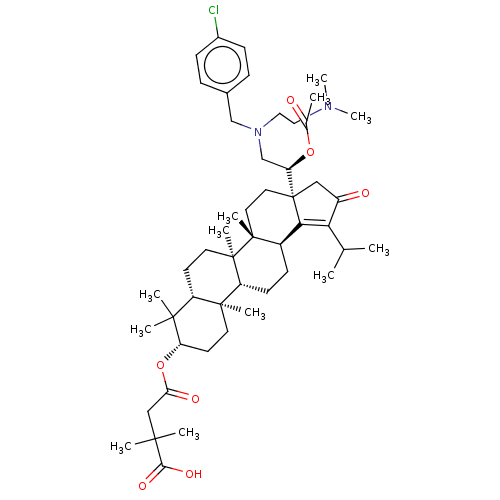
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 41.1nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 34.6nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 5.20nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 4.70nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 61.7nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 243nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 10.1nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 9.30nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 2.60nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 3.20nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 0.5nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 3.80nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 6.10nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 23.1nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 5.90nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 14.3nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 12.3nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 13.5nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 2.30nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 2.40nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 6.30nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 6.70nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 4.5nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 5.10nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 1.60nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 2.10nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 2.10nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 3.60nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 6.90nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 31.1nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 11nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 7.60nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 8.70nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 29.2nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 0.800nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 0.700nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 3.90nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 4.10nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 2.30nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 4.10nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 76.5nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 104nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 3.30nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 3.5nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 4.90nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 11.7nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 40.4nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 1.53E+3nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
TargetEnvelope glycoprotein gp160(Human immunodeficiency virus type 1 (isolate BRU/L...)
Glaxosmithkline
US Patent
Glaxosmithkline
US Patent
Affinity DataEC50: 14.7nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair
Affinity DataEC50: 27.8nMAssay Description:Antiviral HIV activity and compound-induced cytotoxicity were measured in parallel by means of a propidium iodide based procedure in the human T-cell...More data for this Ligand-Target Pair