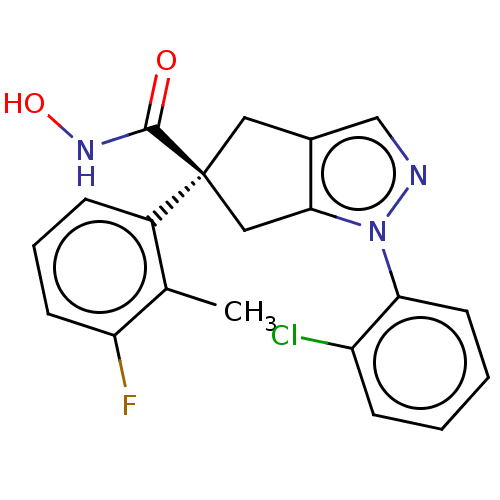
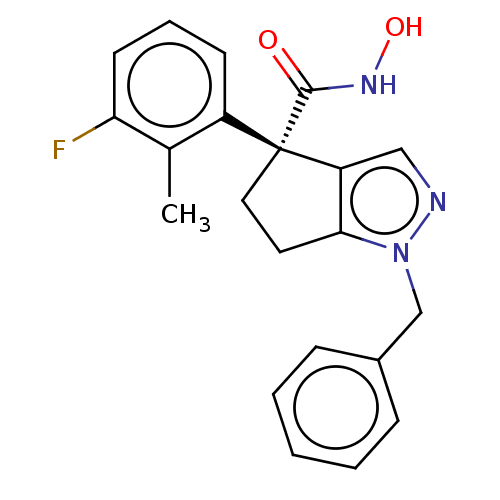
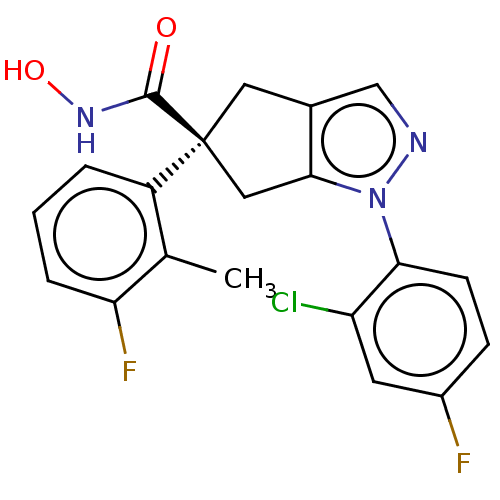
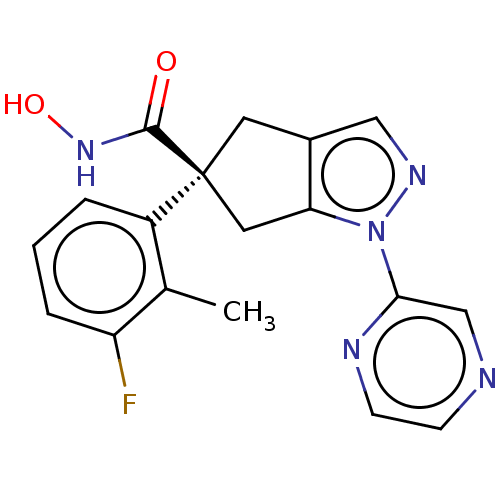
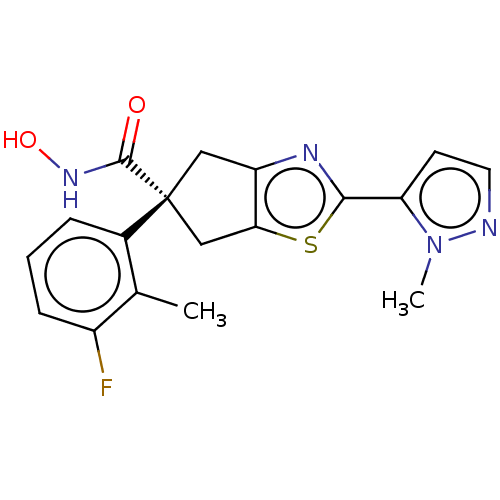
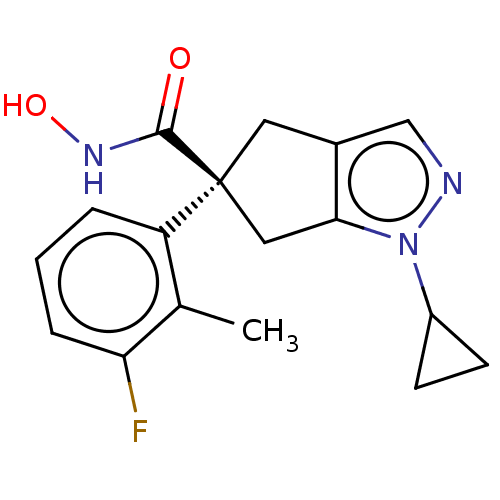
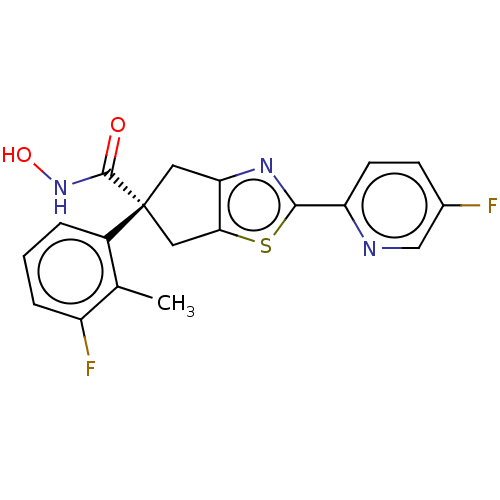
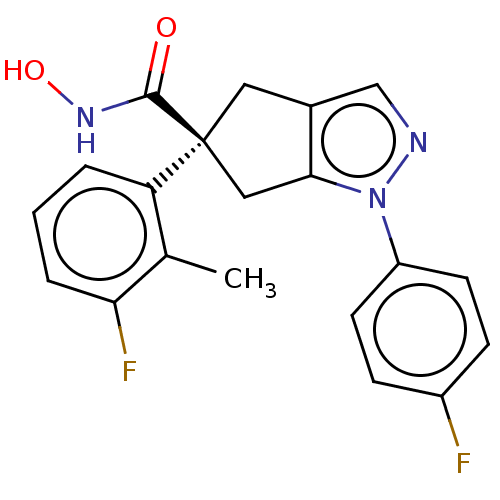
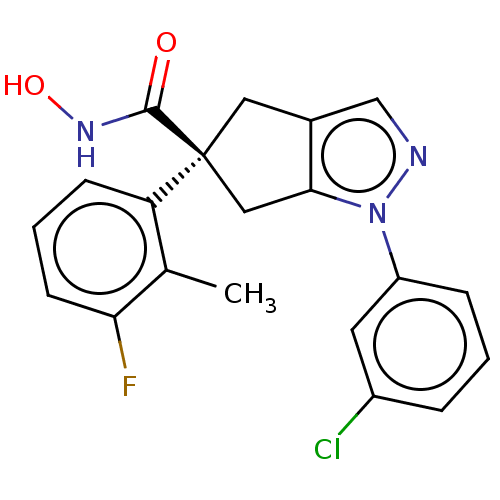
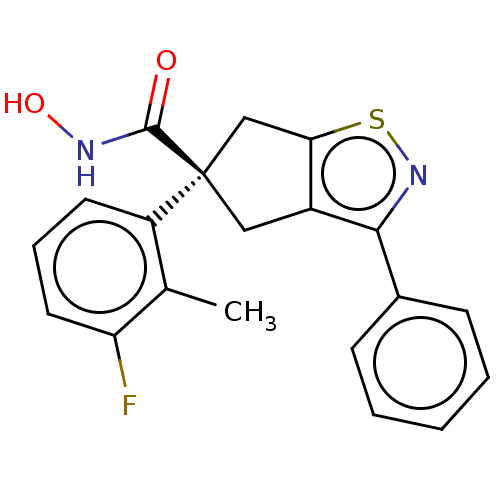
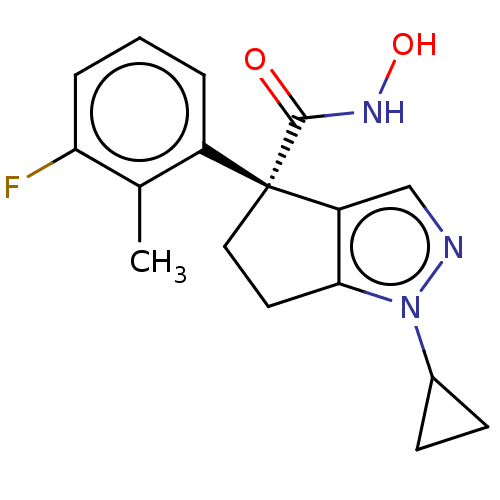
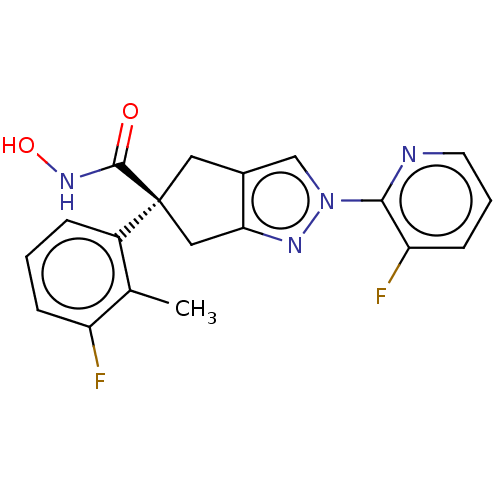
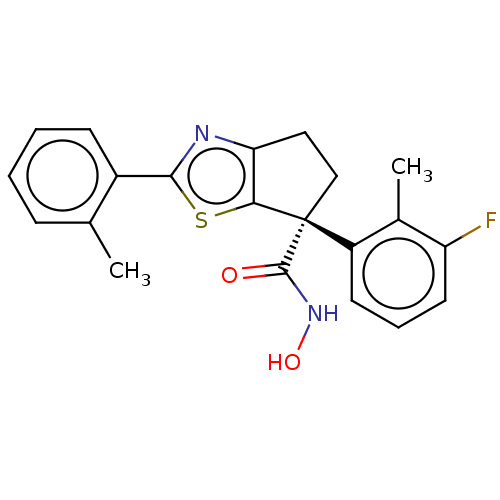
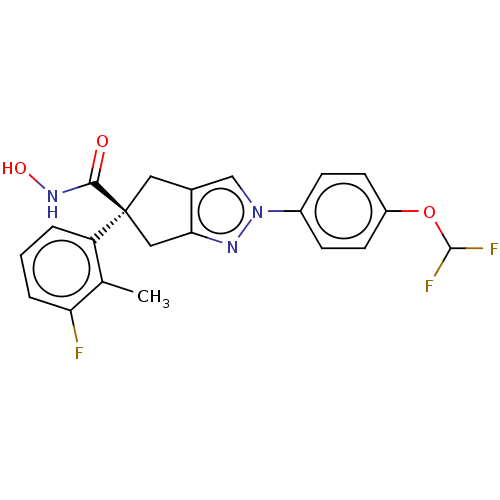
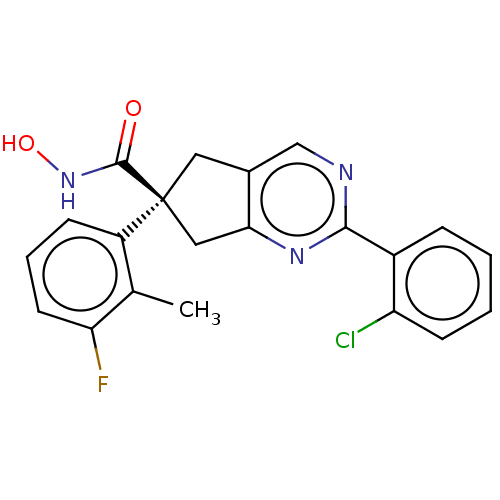
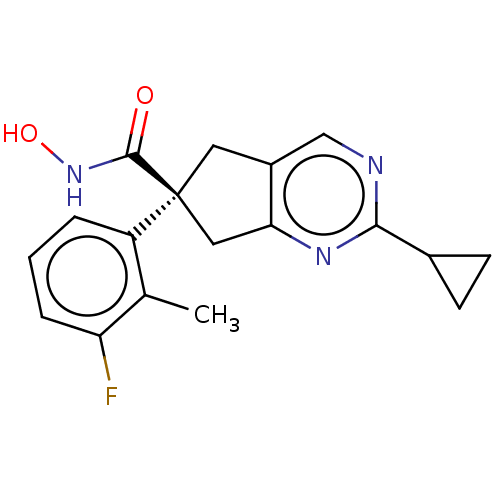
Affinity DataIC50: 12nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 37nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 39nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 44nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 44nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 44nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 48nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 48nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 53nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 53nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 53nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 57nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 61nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 65nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 67nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 68nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 72nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 76nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 79nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 81nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 94nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 95nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 102nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 104nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 109nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 112nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 122nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 143nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 147nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 156nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys...More data for this Ligand-Target Pair