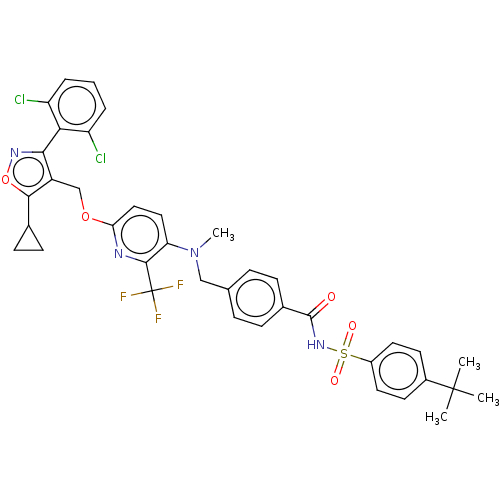
Affinity DataEC50: >1.00E+3nMAssay Description:Determination of a ligand mediated Gal4 promoter driven transactivation to quantify ligand binding mediated activation of FXR. FXR Reporter Assay kit...More data for this Ligand-Target Pair
Affinity DataEC50: <100nMAssay Description:Determination of a ligand mediated Gal4 promoter driven transactivation to quantify ligand binding mediated activation of FXR. FXR Reporter Assay kit...More data for this Ligand-Target Pair
Affinity DataEC50: 5.50E+3nMAssay Description:Determination of a ligand mediated Gal4 promoter driven transactivation to quantify ligand binding mediated activation of FXR. FXR Reporter Assay kit...More data for this Ligand-Target Pair
Affinity DataEC50: 550nMAssay Description:Determination of a ligand mediated Gal4 promoter driven transactivation to quantify ligand binding mediated activation of FXR. FXR Reporter Assay kit...More data for this Ligand-Target Pair

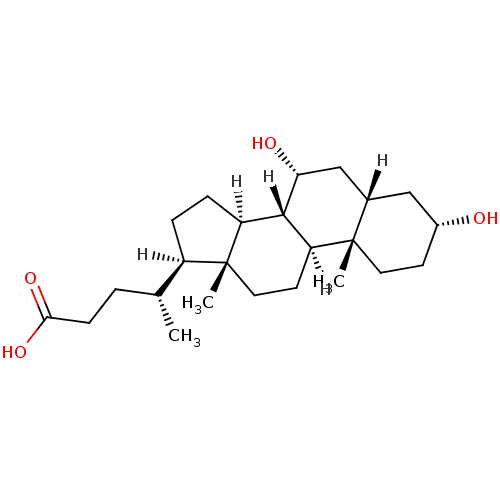
 3D Structure (crystal)
3D Structure (crystal)