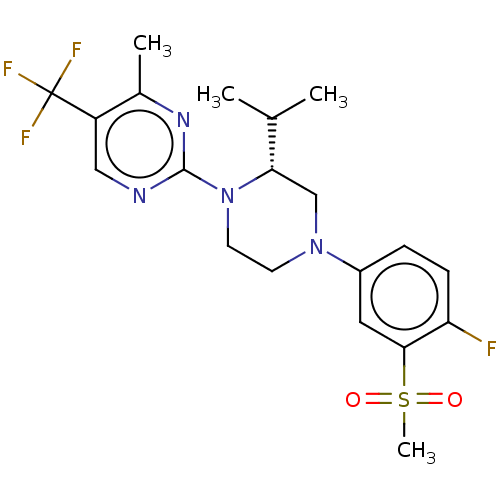
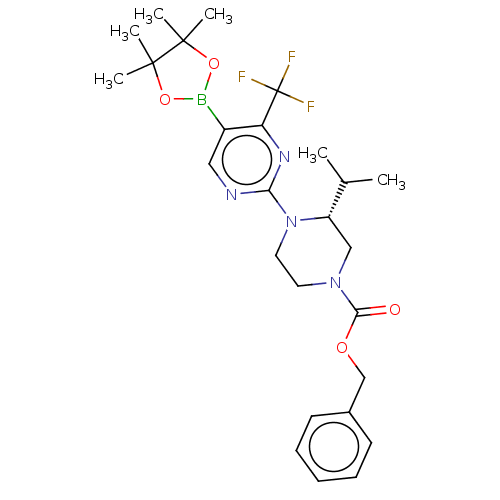
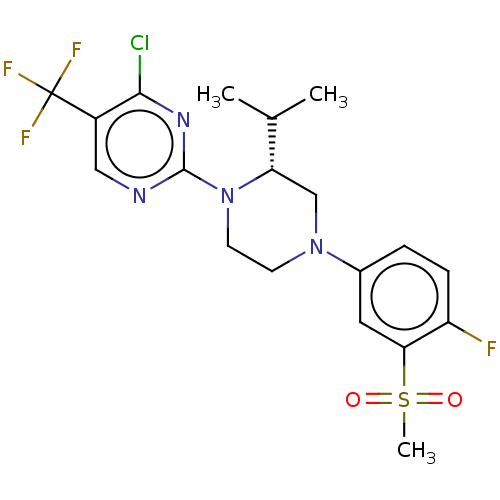
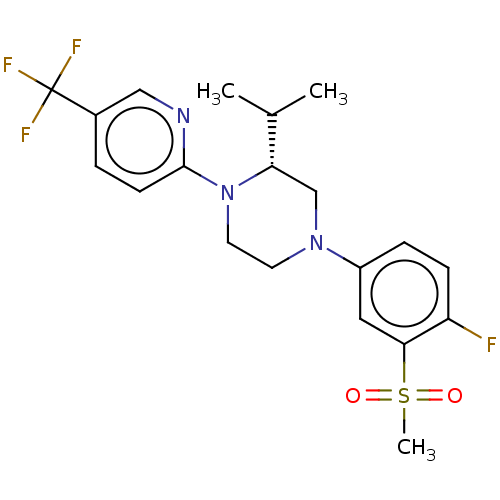
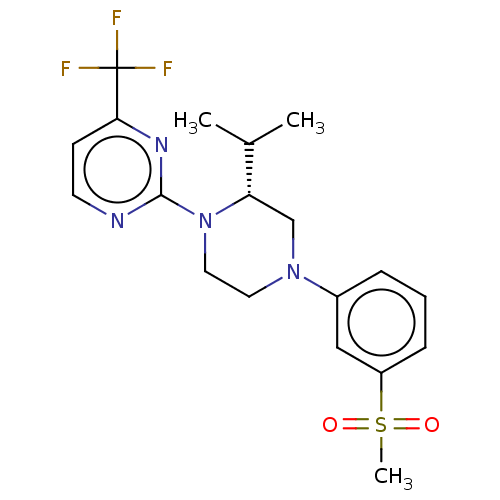
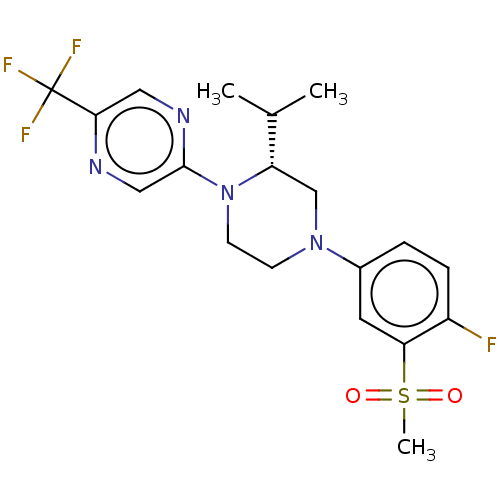
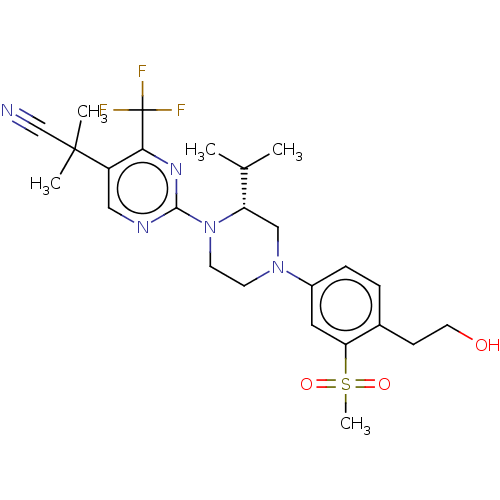
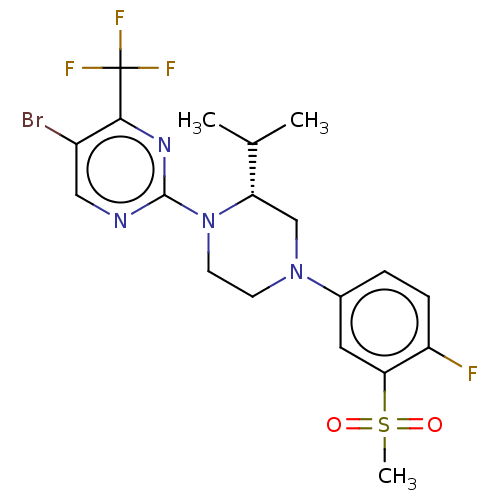
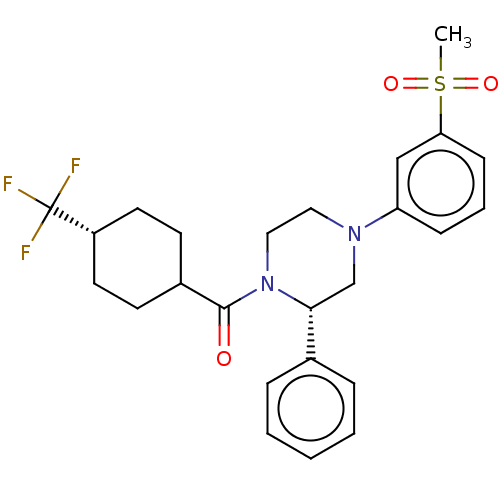
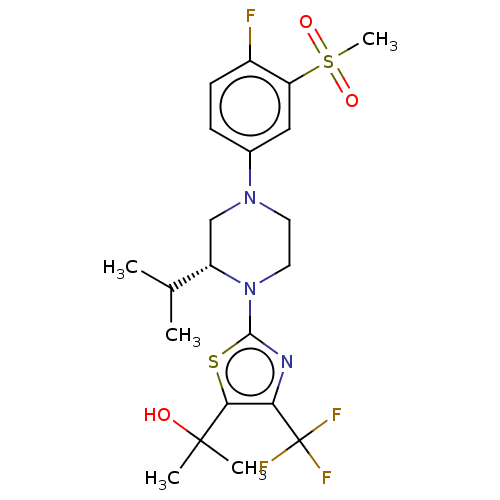
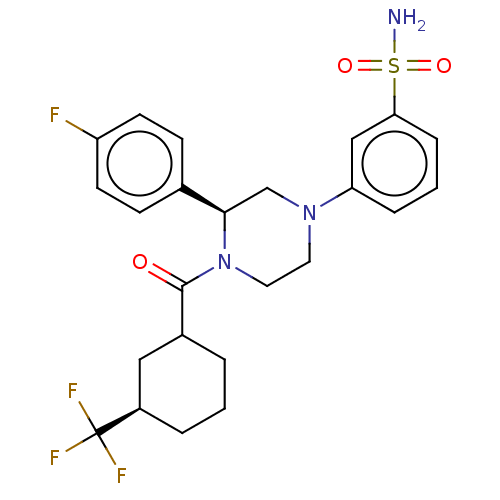
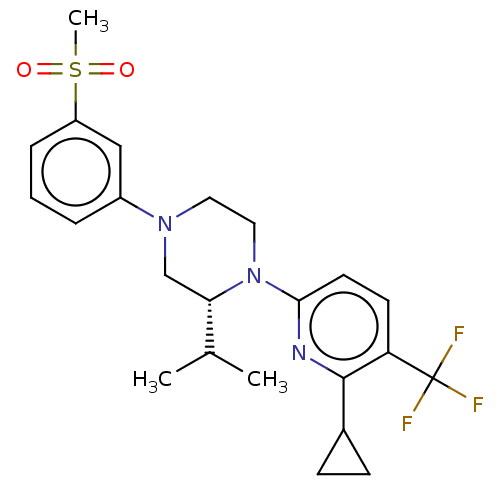
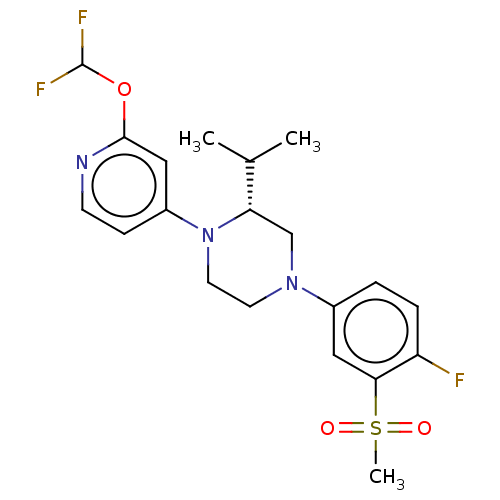
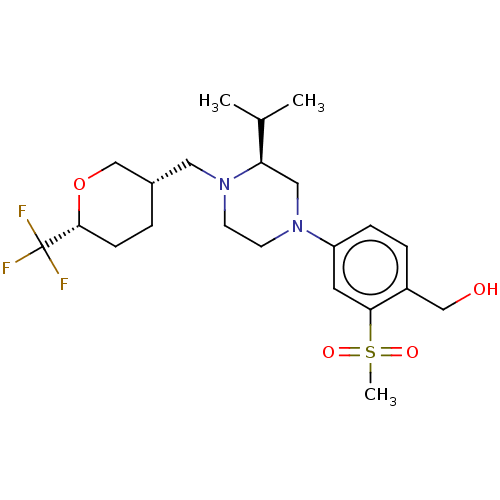
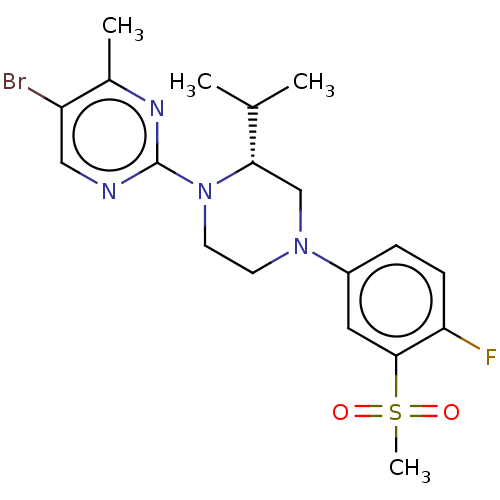
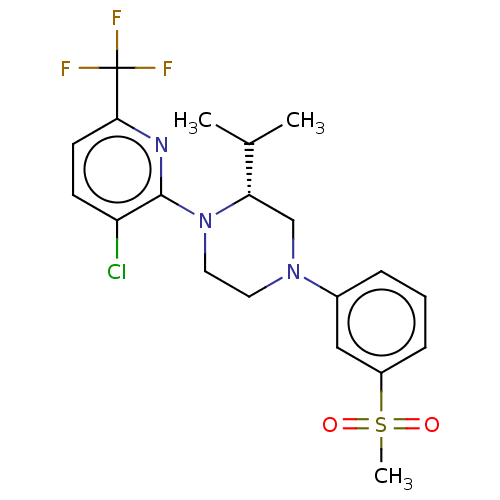
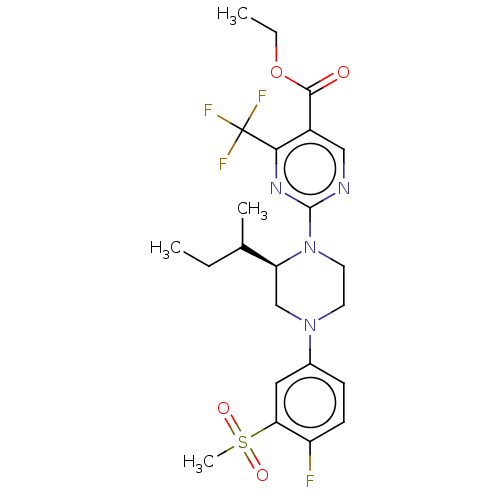
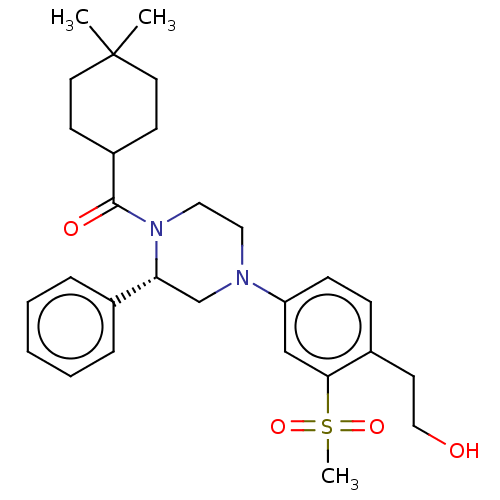
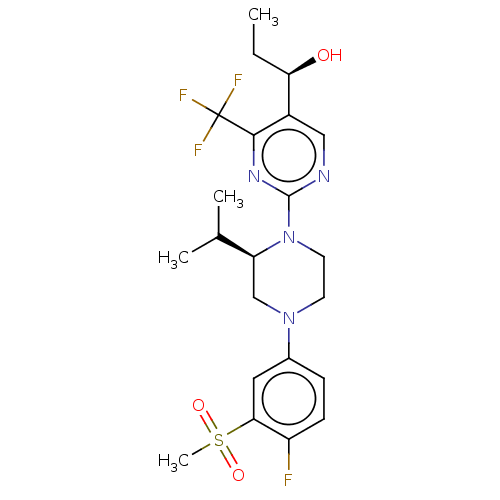
Affinity DataKi: 1nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 6nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair
Affinity DataKi: 9nMAssay Description:Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan...More data for this Ligand-Target Pair


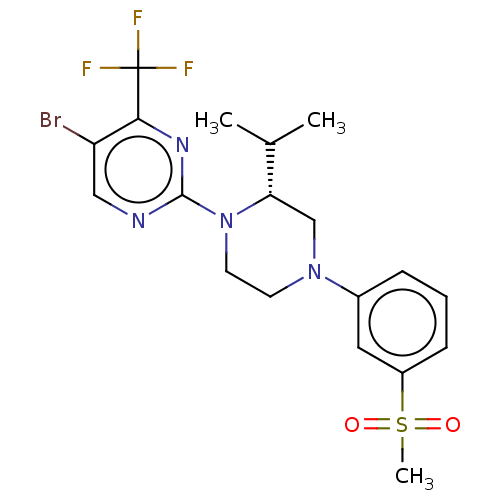
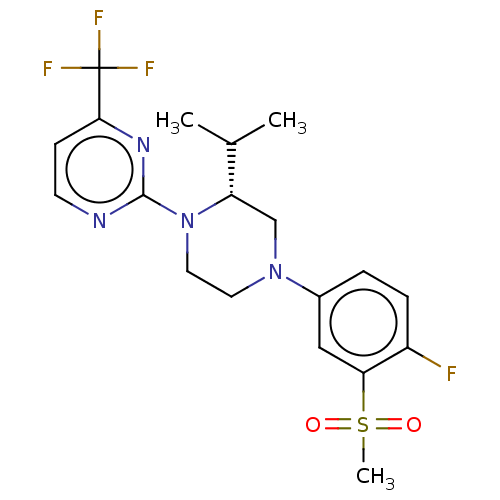
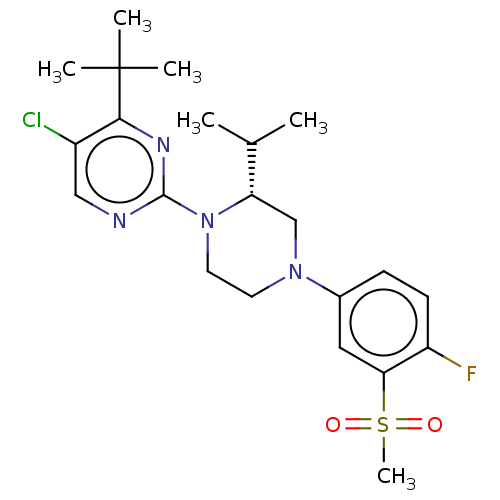
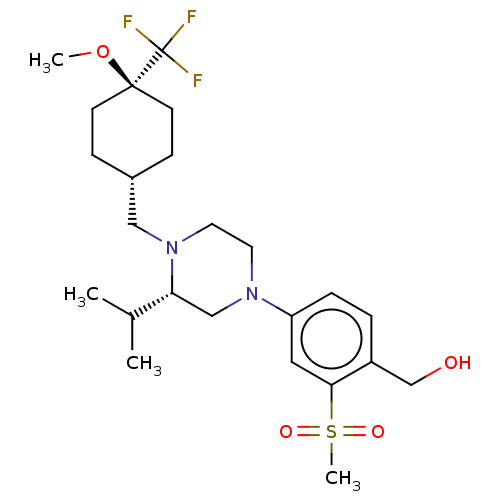
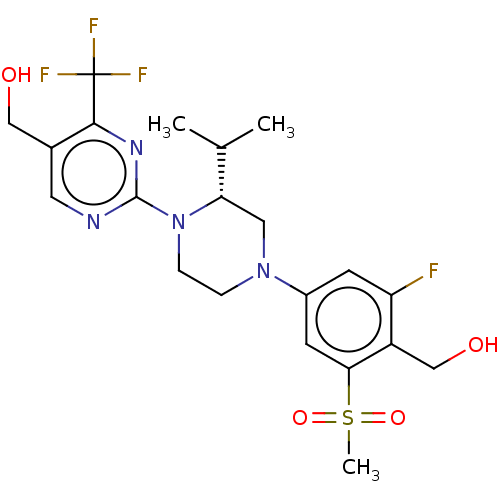
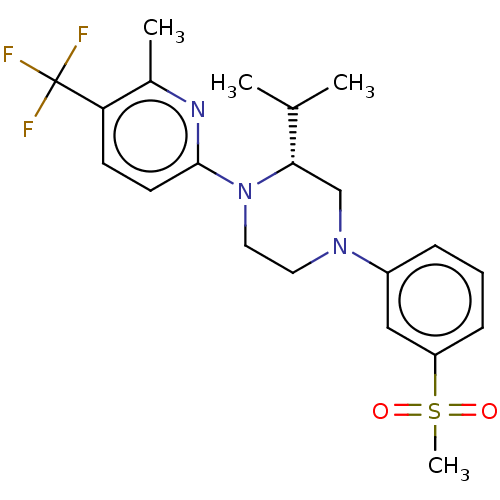
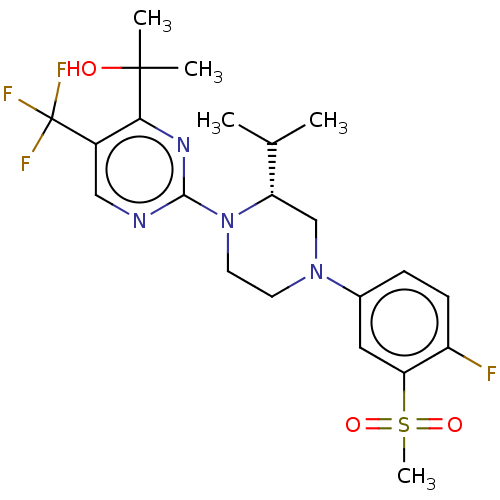
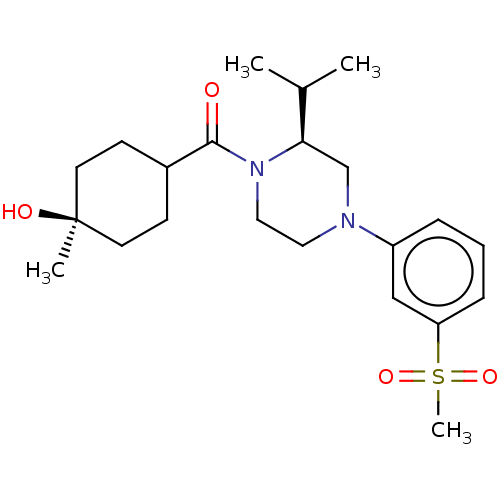
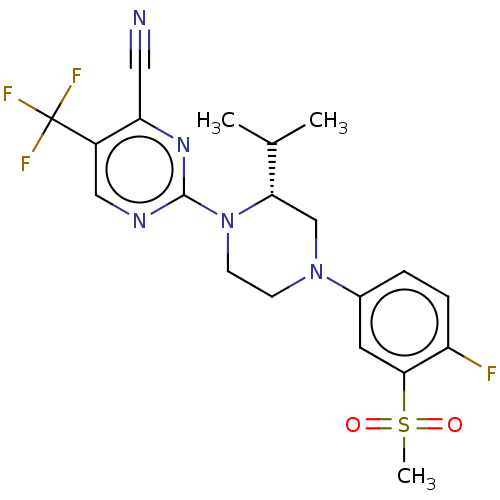
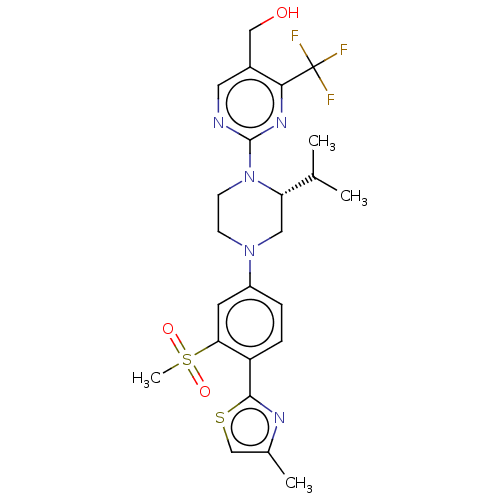

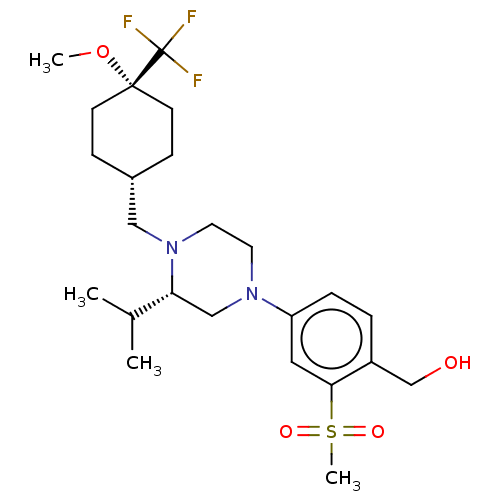
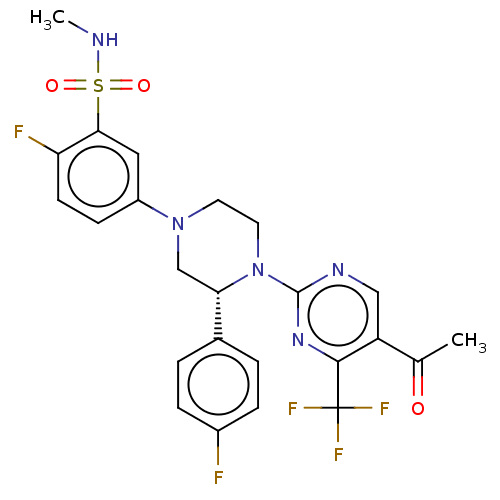
 3D Structure (crystal)
3D Structure (crystal)