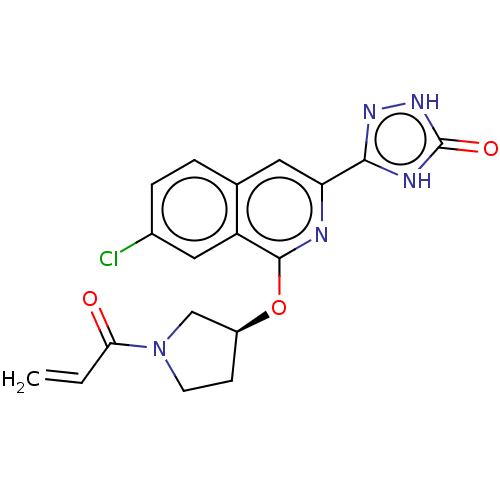
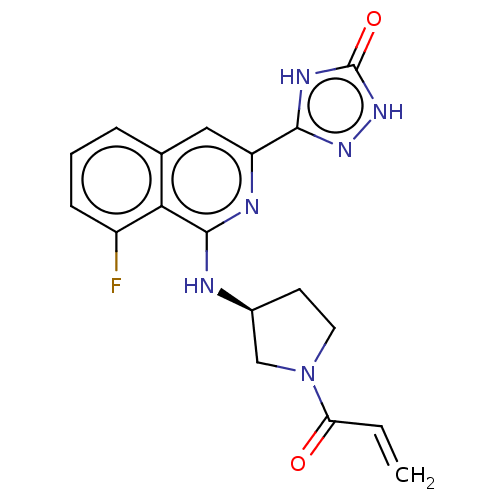
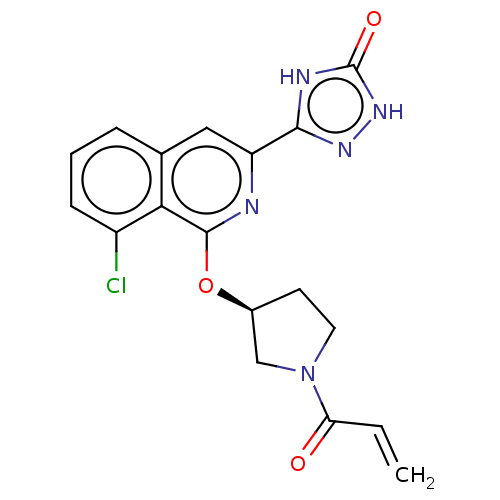
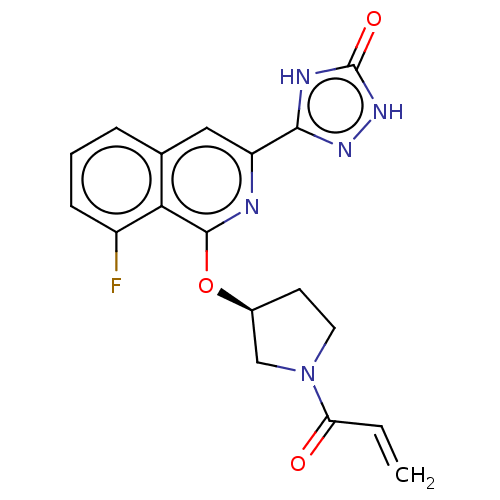
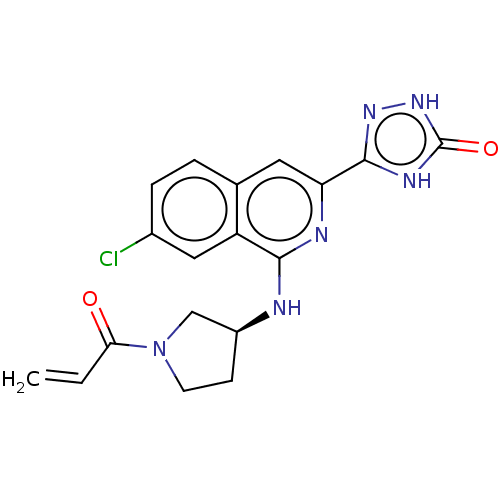
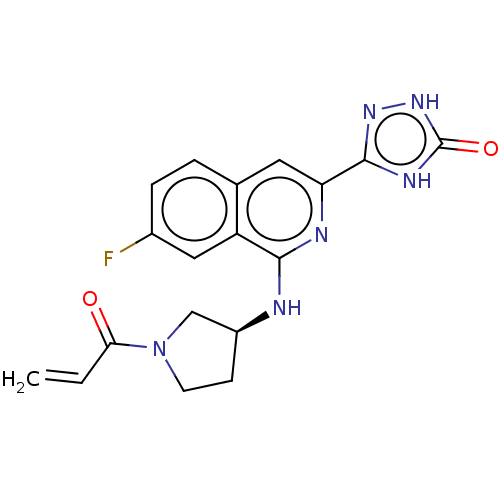
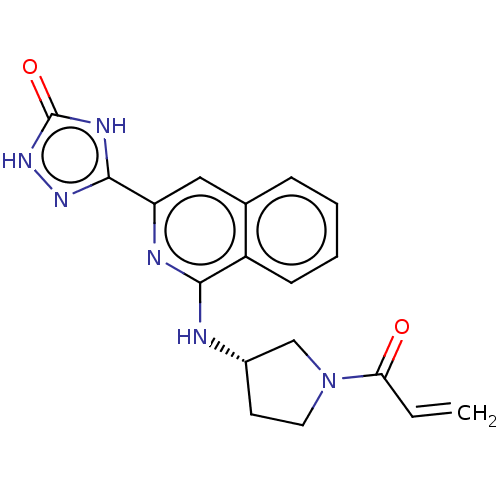
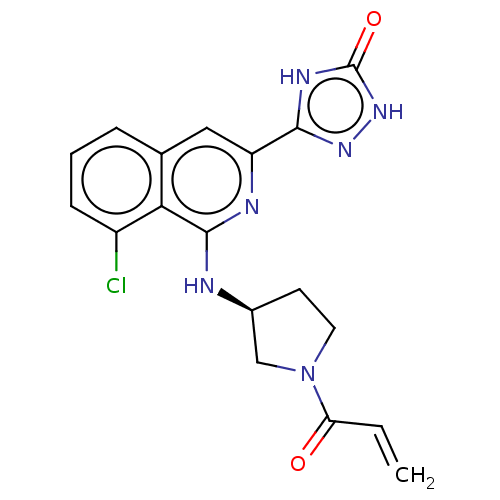
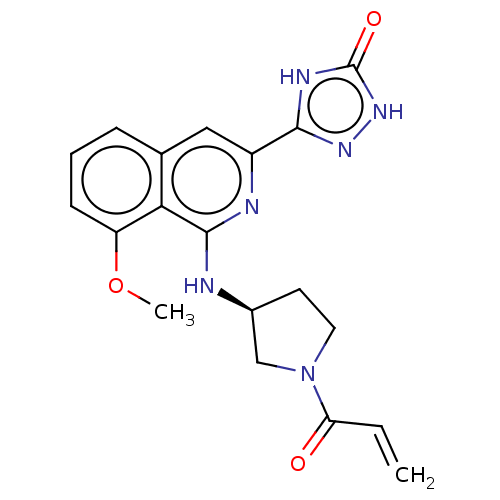
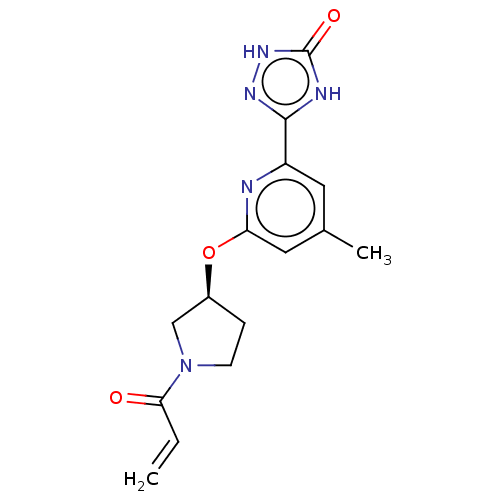
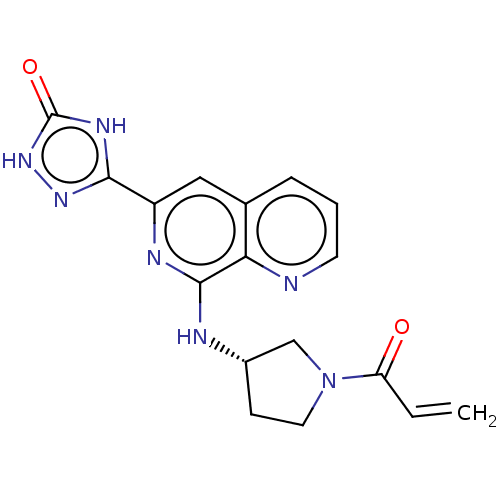
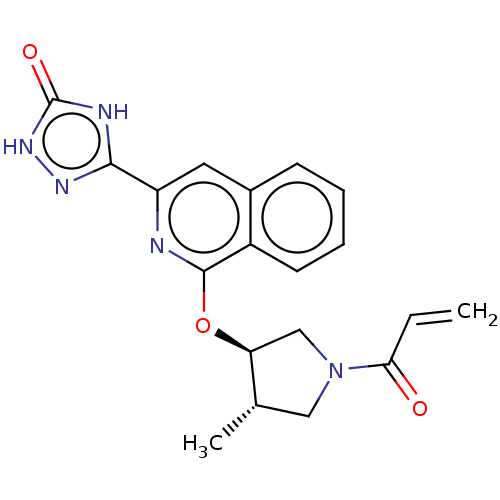
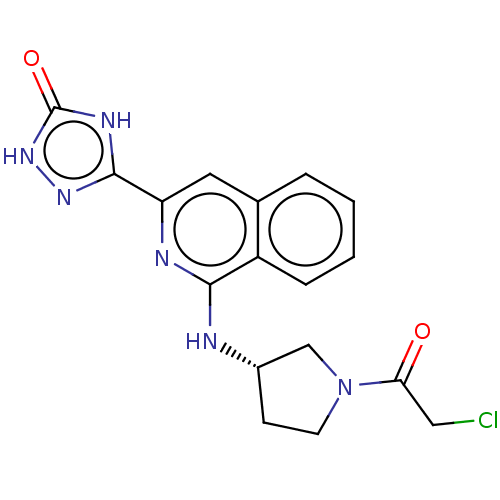
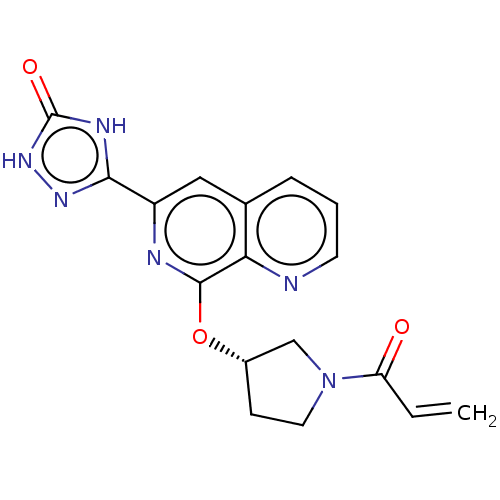
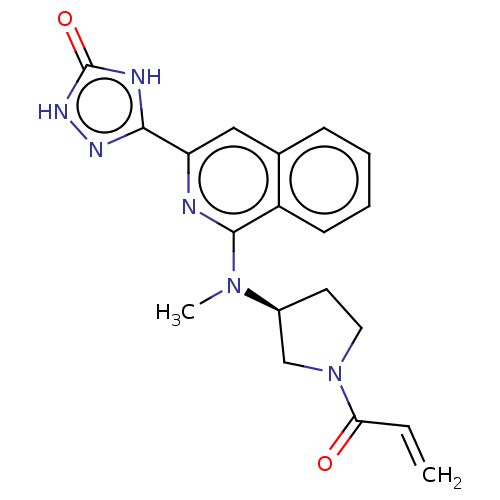
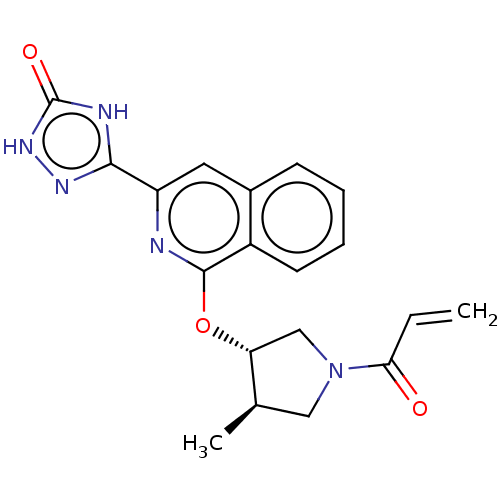
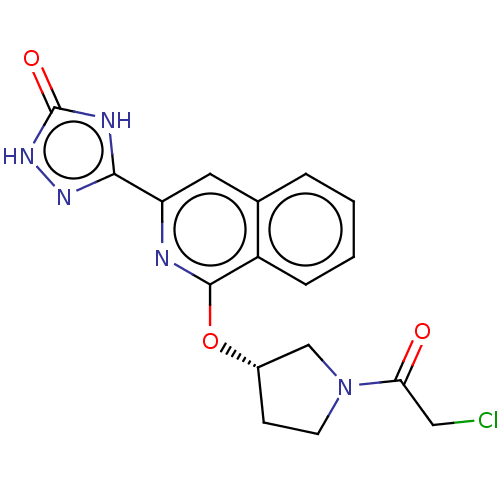
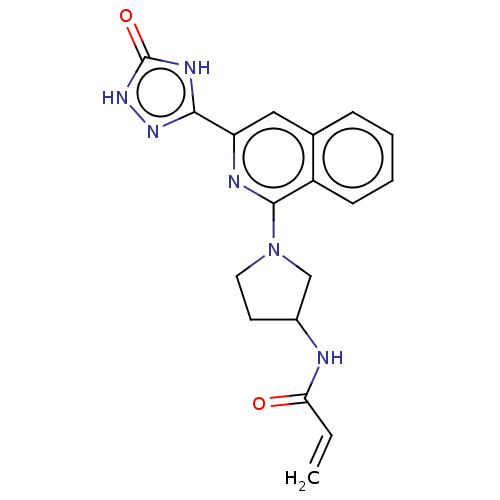
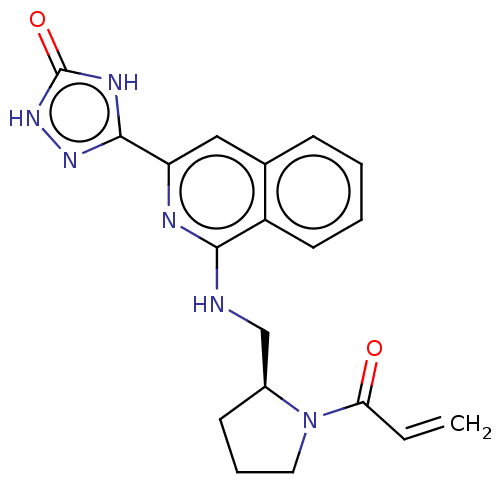
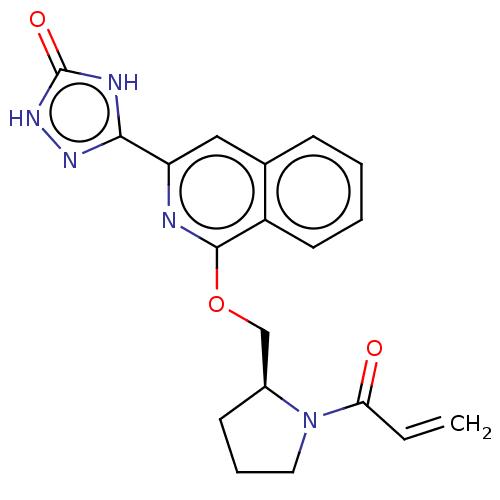
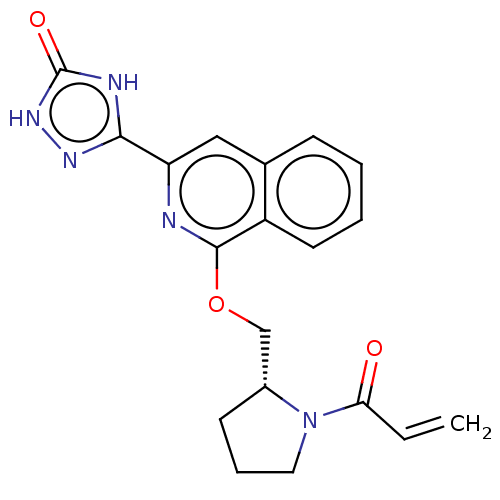
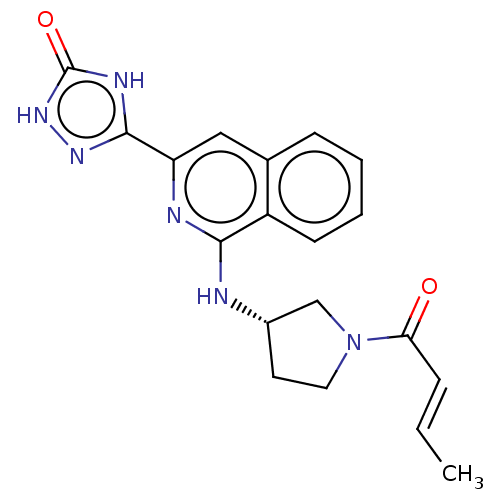
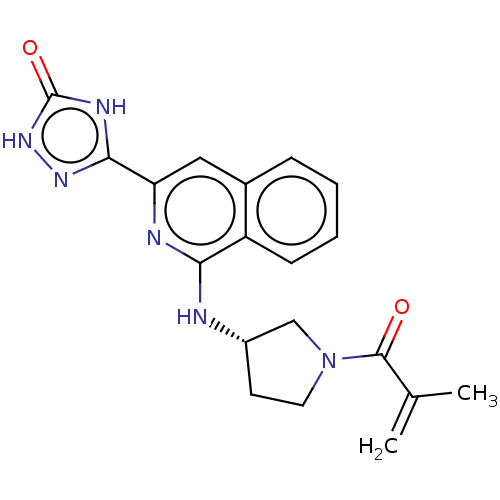
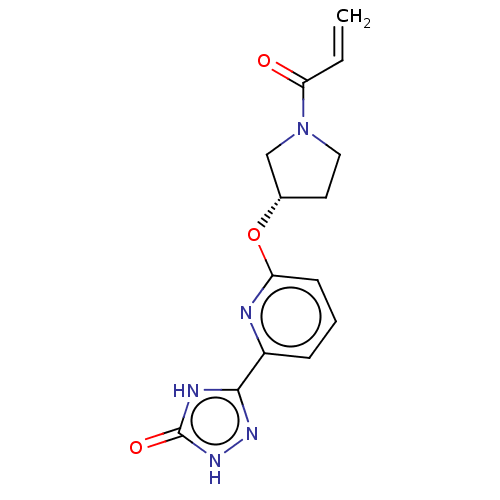
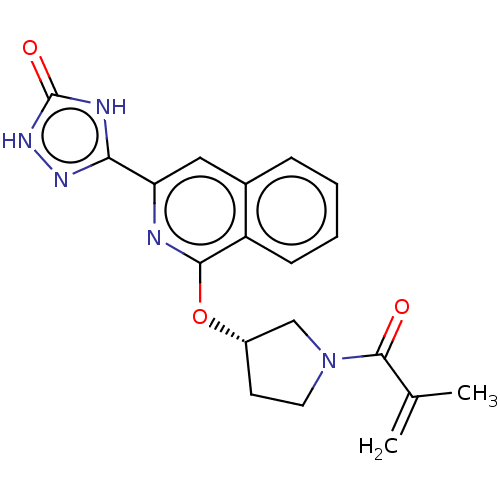
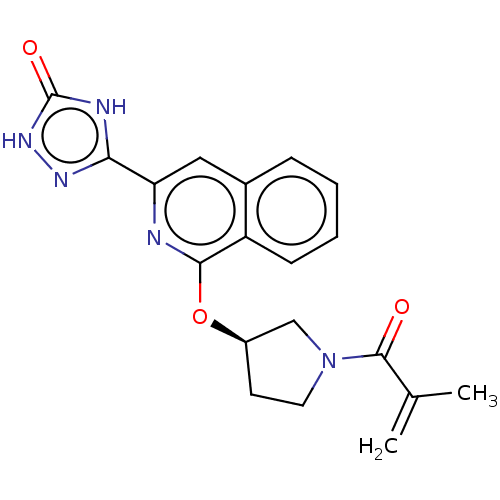
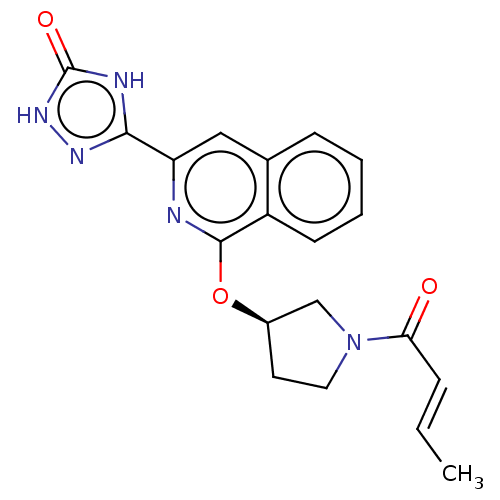
Affinity DataIC50: <1.26nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <1.26nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <1.26nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <1.26nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <2.51nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <3.16nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 3.16nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 5.01nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <6.31nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 6.31nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <6.31nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <6.31nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <7.94nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: <12.6nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 25.1nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 31.6nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 31.6nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 63.1nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 79.4nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 126nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 126nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair
Affinity DataIC50: 316nMAssay Description:The inhibitory properties of compounds relative to BTK is determined using a black 384-well-plate format in a buffer which contains 50 mM Hepes, 10 m...More data for this Ligand-Target Pair