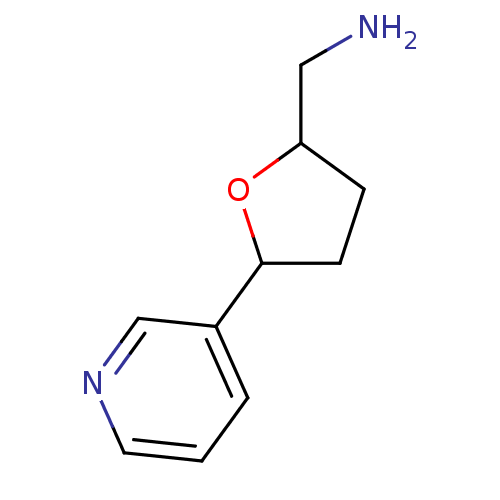
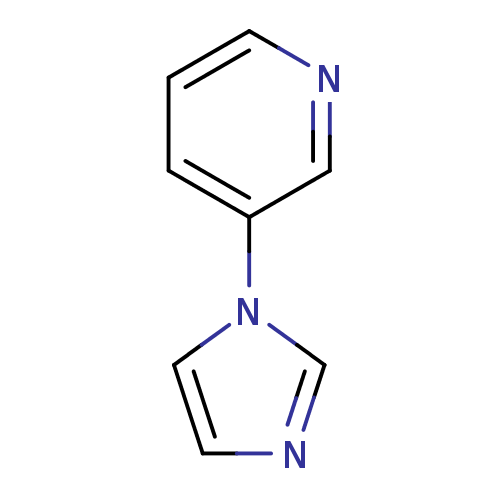
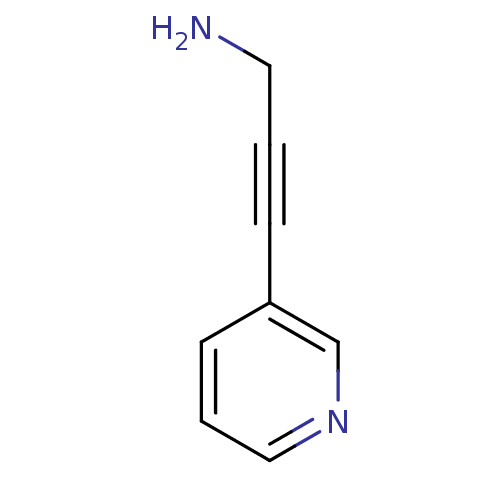
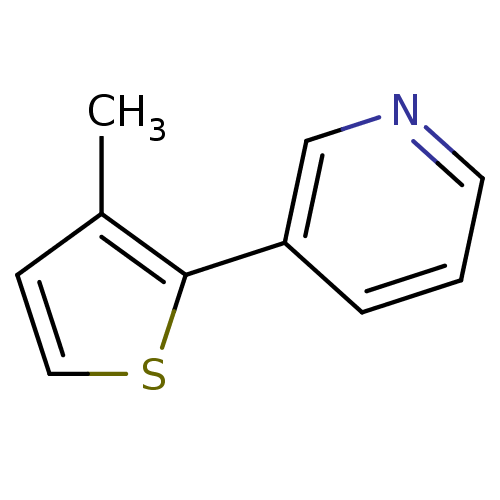
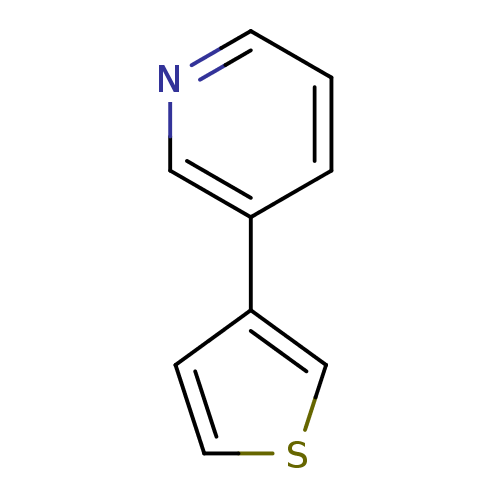
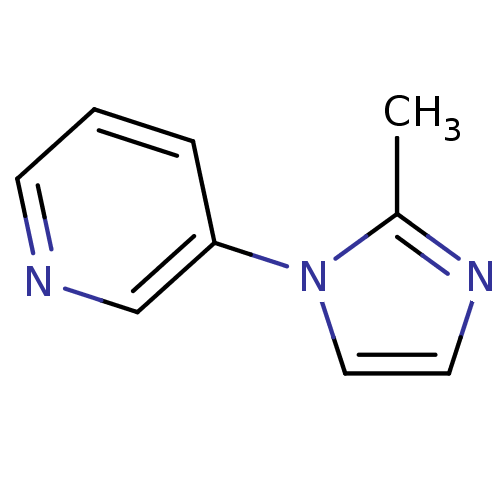
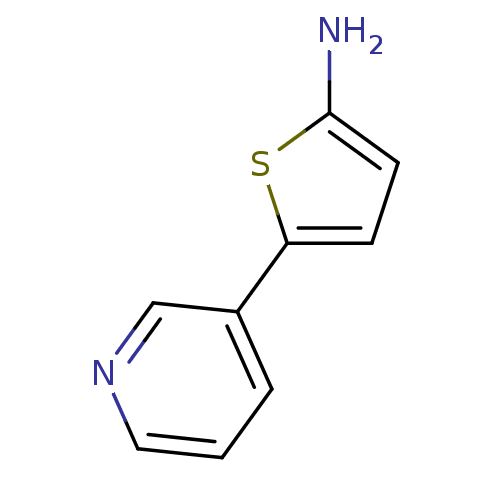
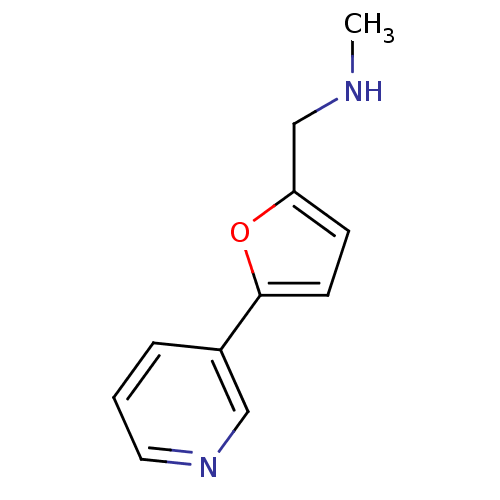
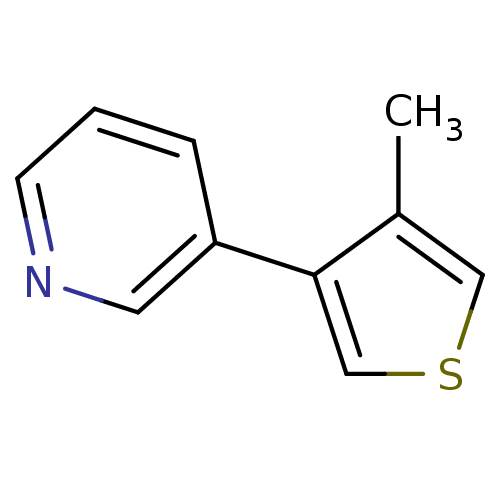
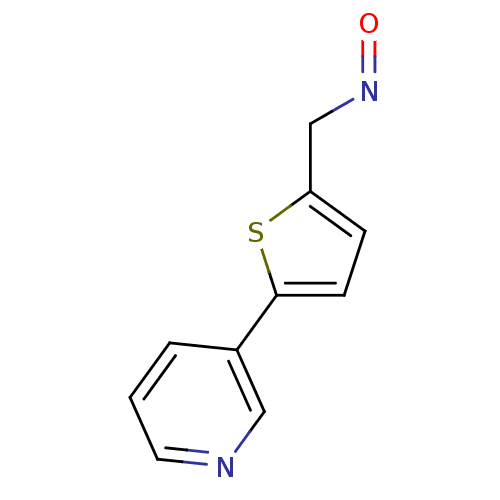
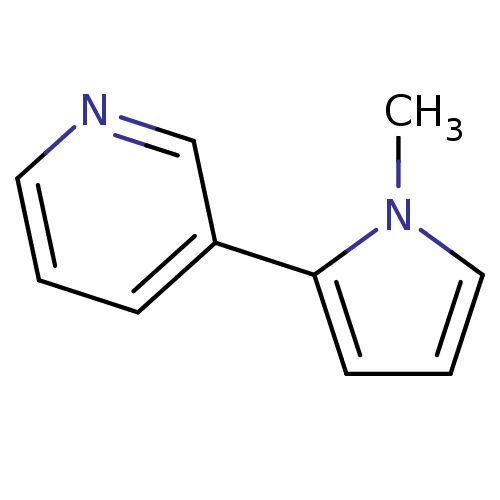
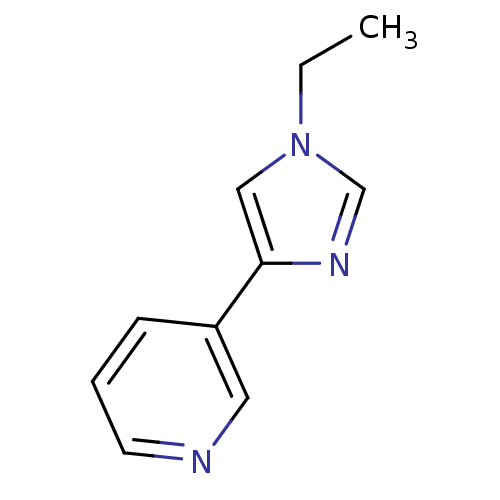
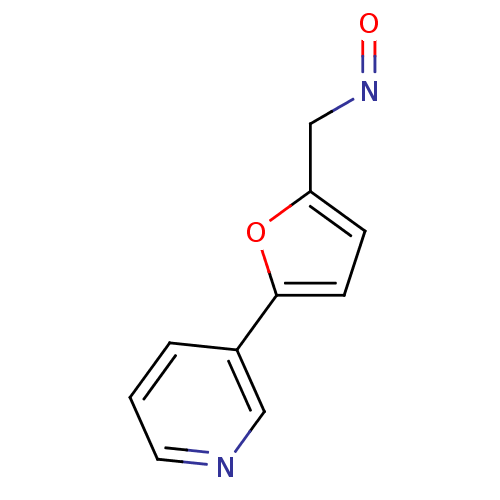
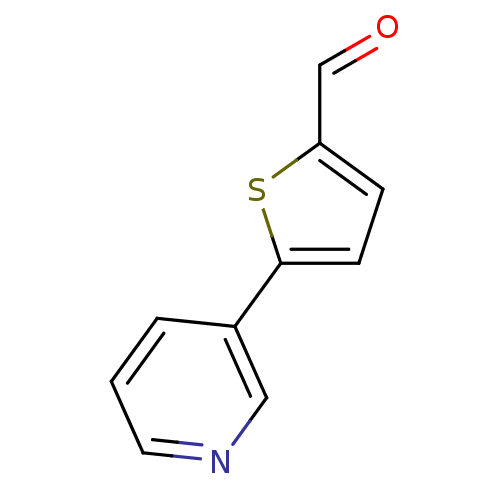
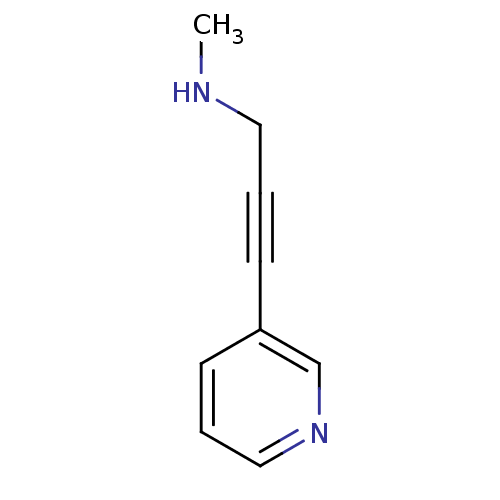
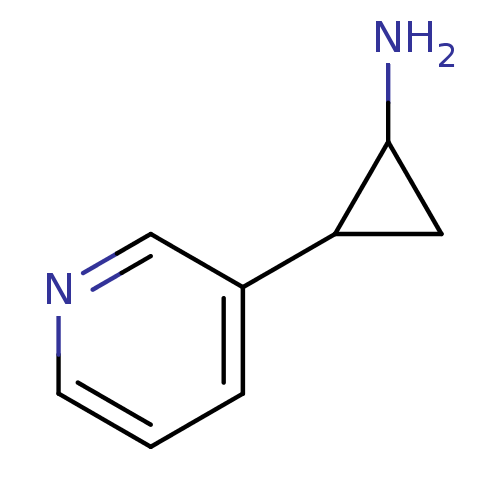
Affinity DataIC50: 20nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 172nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 268nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 330nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 360nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 360nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 514nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 622nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 748nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 970nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 970nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.02E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.02E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.29E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.37E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.39E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.51E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.51E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.59E+3nMAssay Description:To gain insight into the selectivity of the synthetic compounds, nicotine, nicotine related alkaloids and nicotine metabolites for inhibition of othe...More data for this Ligand-Target Pair
Affinity DataIC50: 1.63E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.65E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.85E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.85E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.93E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.05E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.14E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.51E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.74E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.20E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.24E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.77E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.21E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.36E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.77E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.84E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.90E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.90E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.99E+3nMAssay Description:To gain insight into the selectivity of the synthetic compounds, nicotine, nicotine related alkaloids and nicotine metabolites for inhibition of othe...More data for this Ligand-Target Pair
Affinity DataIC50: 6.60E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.60E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 7.44E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 7.65E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 7.65E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 7.80E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair
Affinity DataIC50: 7.80E+3nMAssay Description:The inhibition of human CYP2A6-mediated 7-hydroxy coumarin formation was evaluated in the presence of 95 selected test compounds in a standard assay ...More data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)