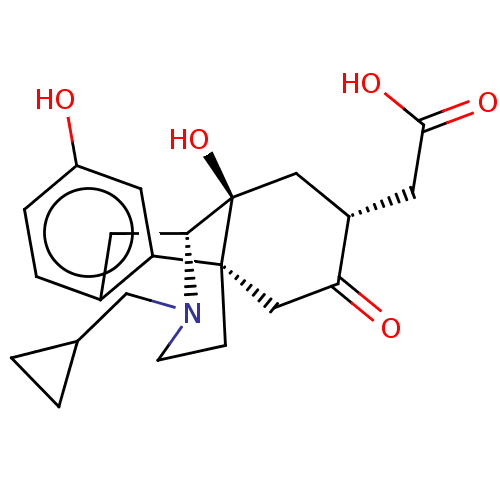
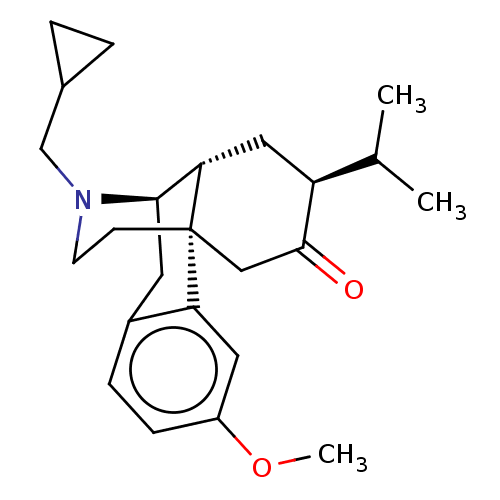
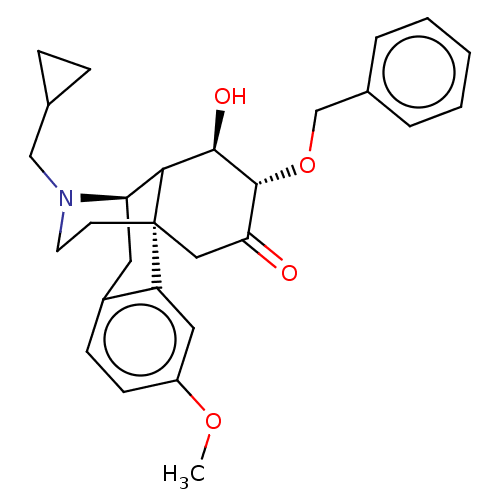
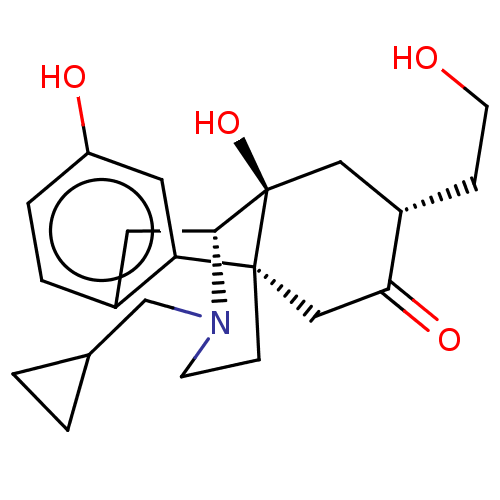
Affinity DataKi: 0.320nM ΔG°: -54.2kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin...More data for this Ligand-Target Pair
Affinity DataKi: 0.330nM ΔG°: -54.1kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin...More data for this Ligand-Target Pair
Affinity DataKi: 0.970nM ΔG°: -51.4kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin...More data for this Ligand-Target Pair
Affinity DataKi: 1.98nM ΔG°: -53.8kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membr...More data for this Ligand-Target Pair
Affinity DataKi: 6.18nM ΔG°: -50.8kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membr...More data for this Ligand-Target Pair
Affinity DataKi: 7.42nM ΔG°: -50.3kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membr...More data for this Ligand-Target Pair
Affinity DataKi: 65.3nM ΔG°: -41.0kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin...More data for this Ligand-Target Pair
Affinity DataKi: 82.9nM ΔG°: -40.4kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin...More data for this Ligand-Target Pair
Affinity DataKi: 128nM ΔG°: -39.3kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin...More data for this Ligand-Target Pair
Affinity DataKi: 146nM ΔG°: -39.0kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin...More data for this Ligand-Target Pair
Affinity DataKi: 191nM ΔG°: -38.4kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin...More data for this Ligand-Target Pair
Affinity DataKi: 223nM ΔG°: -41.2kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membr...More data for this Ligand-Target Pair
Affinity DataKi: 249nM ΔG°: -37.7kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin...More data for this Ligand-Target Pair
Affinity DataKi: 261nM ΔG°: -37.6kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin...More data for this Ligand-Target Pair
Affinity DataKi: 324nM ΔG°: -37.0kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin...More data for this Ligand-Target Pair
Affinity DataKi: 394nM ΔG°: -36.6kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin...More data for this Ligand-Target Pair
Affinity DataKi: 408nM ΔG°: -36.5kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin...More data for this Ligand-Target Pair
Affinity DataKi: 476nM ΔG°: -39.1kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membr...More data for this Ligand-Target Pair
Affinity DataKi: 591nM ΔG°: -38.5kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membr...More data for this Ligand-Target Pair
Affinity DataKi: 668nM ΔG°: -38.2kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membr...More data for this Ligand-Target Pair
Affinity DataKi: 1.96E+3nM ΔG°: -35.3kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membr...More data for this Ligand-Target Pair
Affinity DataKi: 3.41E+3nM ΔG°: -31.2kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin...More data for this Ligand-Target Pair
Affinity DataKi: 1.59E+4nM ΔG°: -27.4kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin...More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+4nM ΔG°: >-26.8kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin...More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+4nM ΔG°: >-26.8kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin...More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+4nM ΔG°: >-26.8kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin...More data for this Ligand-Target Pair
Affinity DataEC50: 3.40E+3nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: 703nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: >2.00E+5nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: 1.47E+4nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: >2.00E+5nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: 4.60E+3nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: 43.8nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: 6.77E+3nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: >2.00E+5nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: 2.75E+3nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: 49.7nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: >2.00E+5nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: >2.00E+5nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: 463nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: 14.2nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: >2.00E+5nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: 212nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: 2.85nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: 2.31nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: 2.33E+3nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: 457nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: 1.26E+3nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: 2.10E+3nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair
Affinity DataEC50: 463nMpH: 7.4 T: 2°CAssay Description:[35S]GTPγS functional assays were conducted using freshly thawed μ-receptor membranes prepared from a cell line expressing recombinant μ ...More data for this Ligand-Target Pair