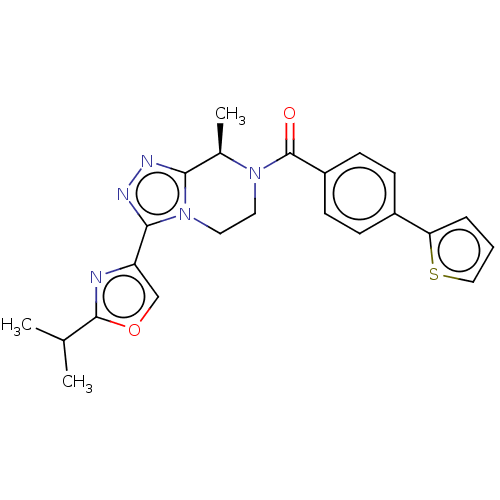
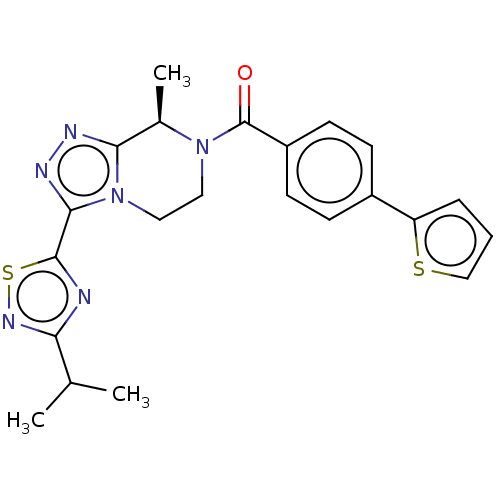
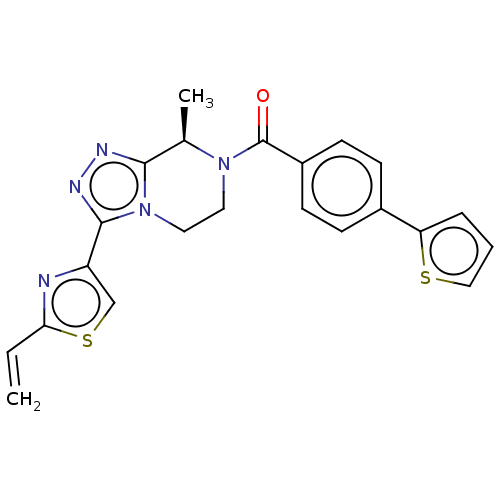
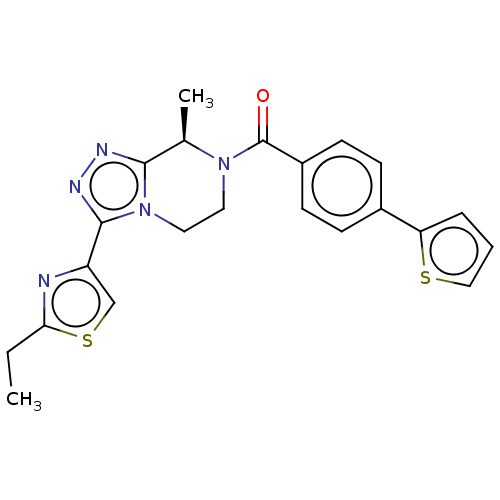
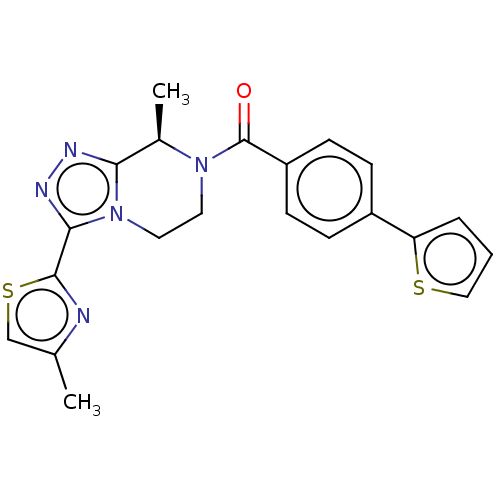
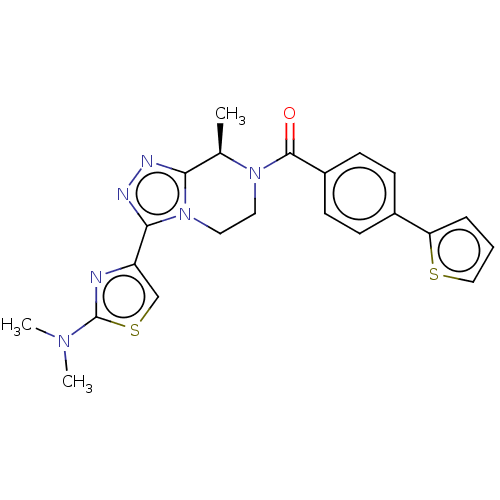
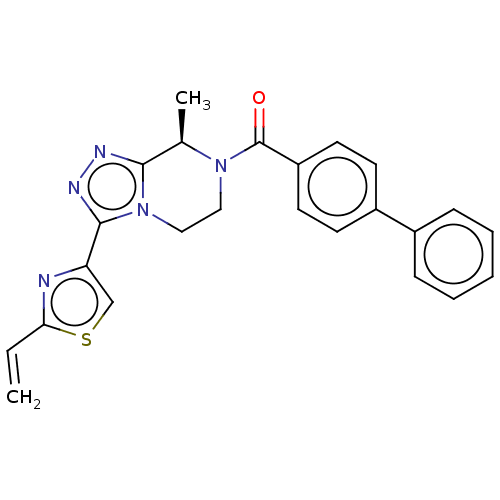
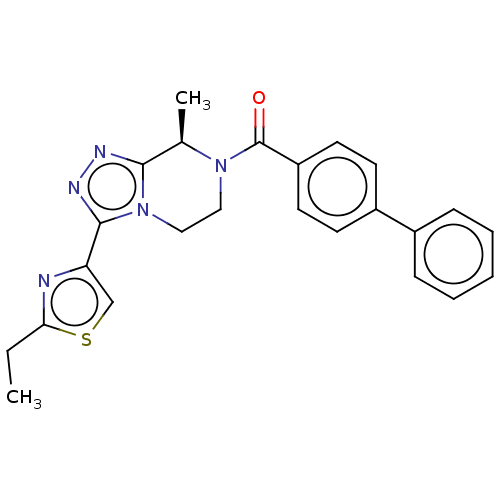
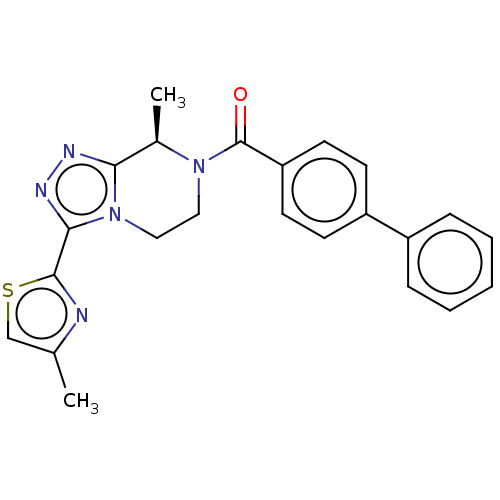
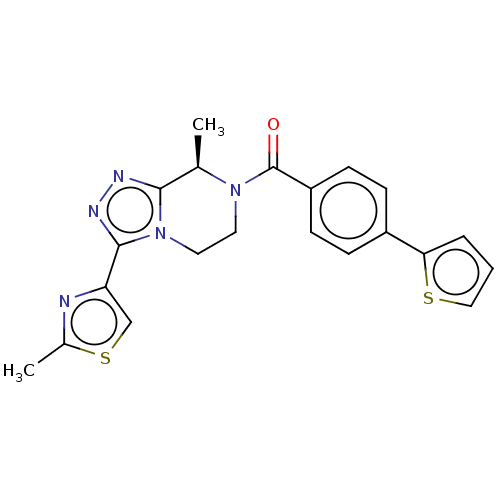
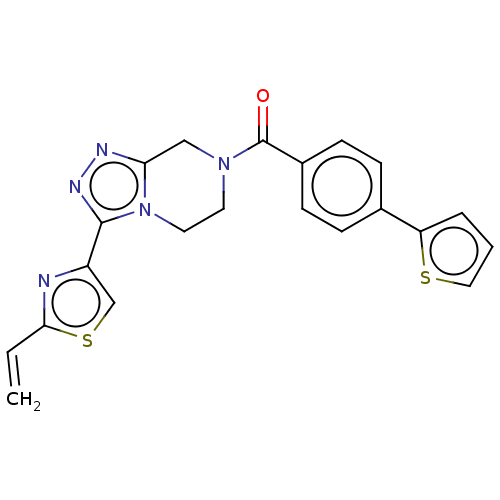
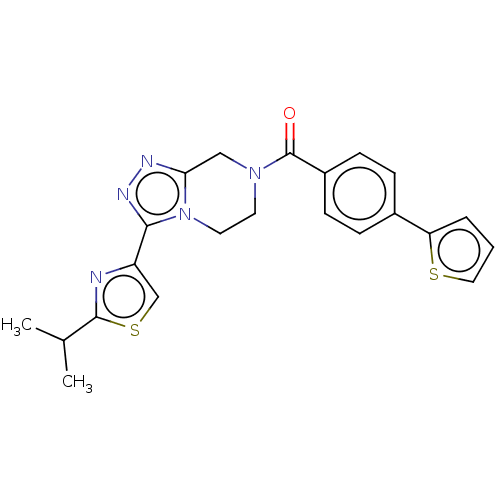
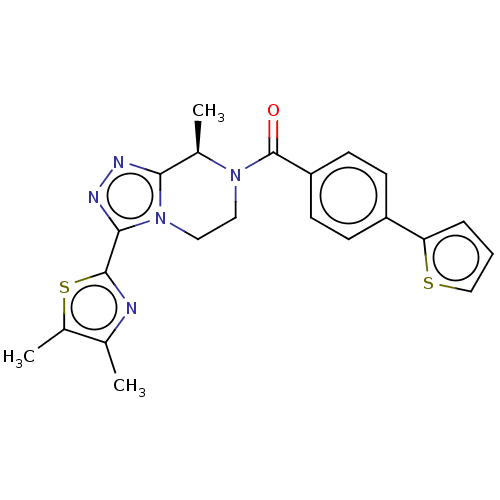
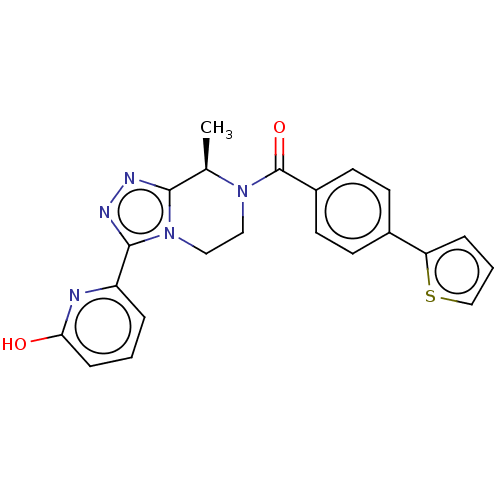
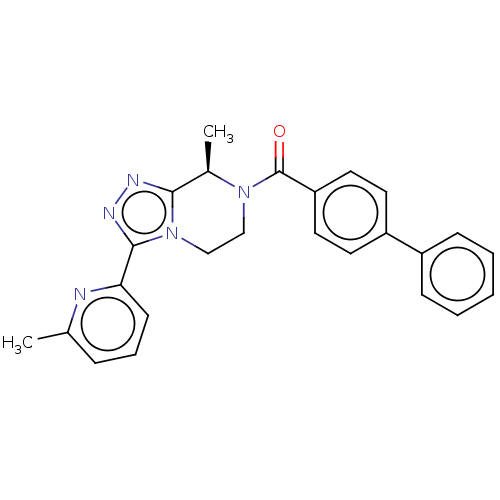
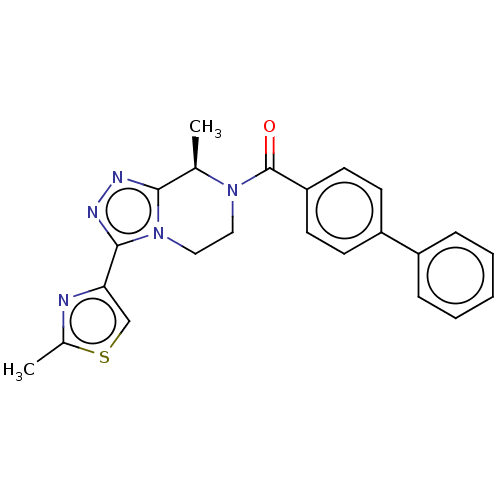
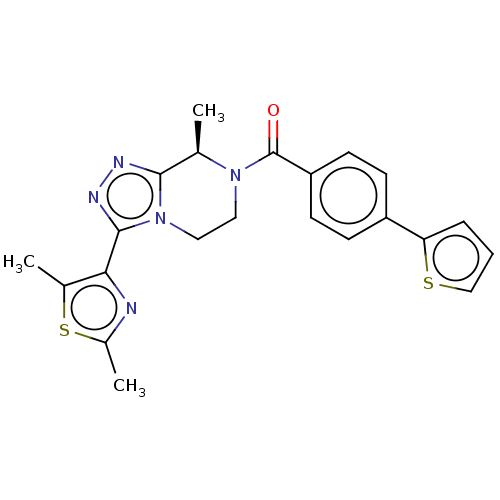
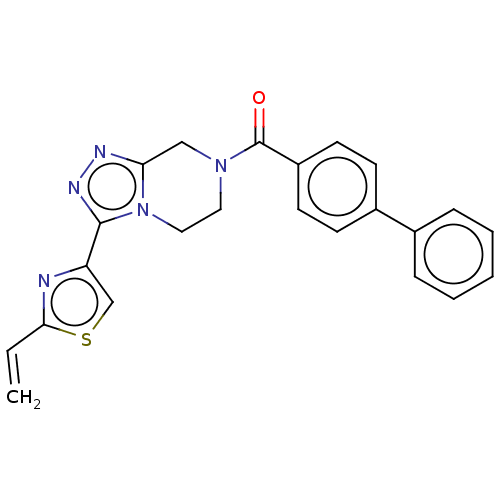
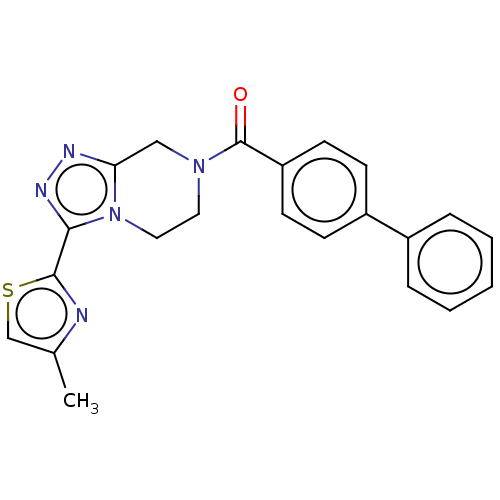
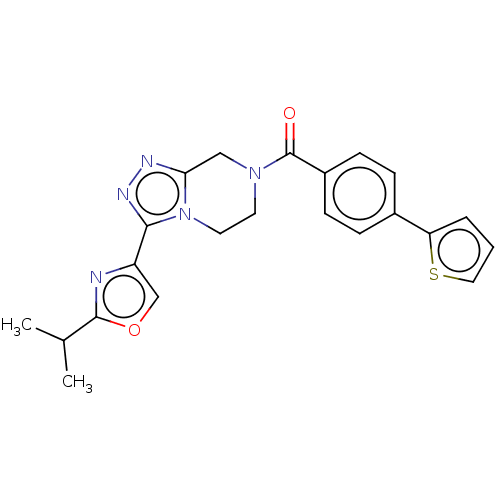
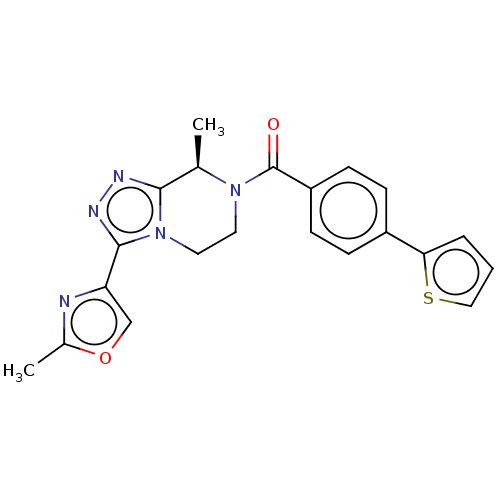
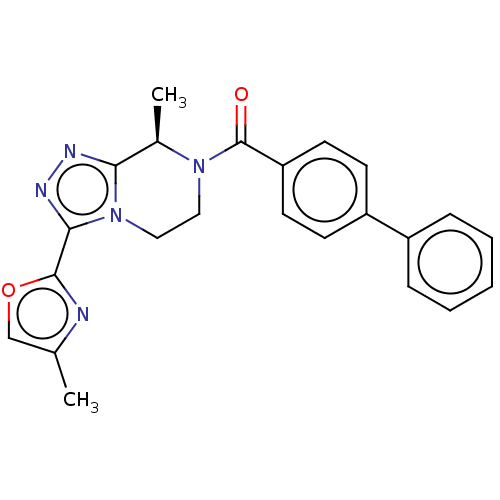
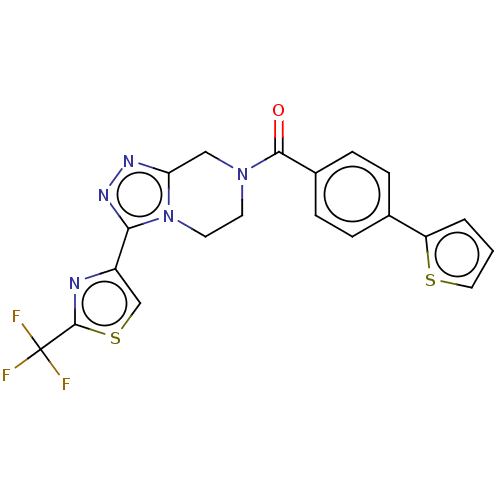
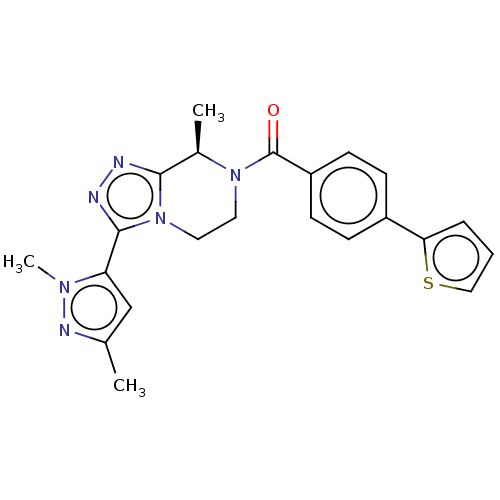
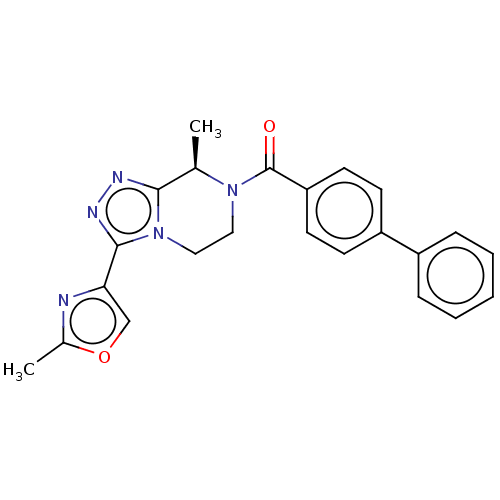
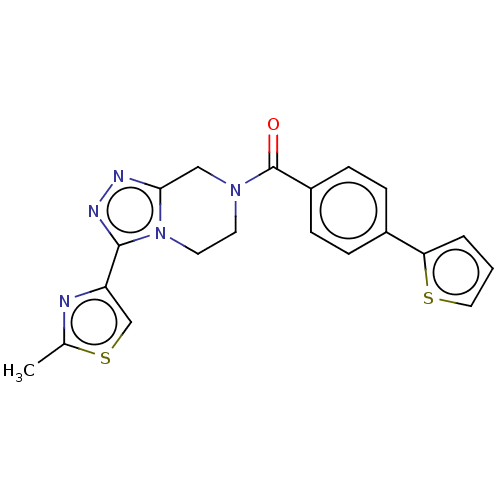
Affinity DataKi: 3nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 3nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 4nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 5nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 6nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 7nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 7nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 10nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 11nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 11nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 12nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 16nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 19nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 20nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 21nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 22nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 23nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 26nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 29nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 32nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 36nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 38nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 42nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 44nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 51nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 56nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 68nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 70nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 83nMpH: 7.4Assay Description:The ability of compounds of the invention to inhibit the binding of the NK-3 receptor selective antagonist 3H-SB222200 was assessed by an in vitro ra...More data for this Ligand-Target Pair
Affinity DataKi: 5.93E+3nM ΔG°: -29.8kJ/molepH: 7.4 T: 2°CAssay Description:The affinity of compounds of the invention for the NK2 receptor was evaluated in CHO recombinant cells which express the human NK2 receptor. Membrane...More data for this Ligand-Target Pair
Affinity DataKi: 6.06E+3nM ΔG°: -29.8kJ/molepH: 7.4 T: 2°CAssay Description:The affinity of compounds of the invention for the NK1 receptor was evaluated in CHO recombinant cells which express the human NK1 receptor. Membrane...More data for this Ligand-Target Pair
Affinity DataKi: 9.95E+3nM ΔG°: -28.6kJ/molepH: 7.4 T: 2°CAssay Description:The affinity of compounds of the invention for the NK2 receptor was evaluated in CHO recombinant cells which express the human NK2 receptor. Membrane...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nM ΔG°: >-28.5kJ/molepH: 7.4 T: 2°CAssay Description:The affinity of compounds of the invention for the NK1 receptor was evaluated in CHO recombinant cells which express the human NK1 receptor. Membrane...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nM ΔG°: >-28.5kJ/molepH: 7.4 T: 2°CAssay Description:The affinity of compounds of the invention for the NK2 receptor was evaluated in CHO recombinant cells which express the human NK2 receptor. Membrane...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nM ΔG°: >-28.5kJ/molepH: 7.4 T: 2°CAssay Description:The affinity of compounds of the invention for the NK2 receptor was evaluated in CHO recombinant cells which express the human NK2 receptor. Membrane...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nM ΔG°: >-28.5kJ/molepH: 7.4 T: 2°CAssay Description:The affinity of compounds of the invention for the NK2 receptor was evaluated in CHO recombinant cells which express the human NK2 receptor. Membrane...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nM ΔG°: >-28.5kJ/molepH: 7.4 T: 2°CAssay Description:The affinity of compounds of the invention for the NK1 receptor was evaluated in CHO recombinant cells which express the human NK1 receptor. Membrane...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nM ΔG°: >-28.5kJ/molepH: 7.4 T: 2°CAssay Description:The affinity of compounds of the invention for the NK1 receptor was evaluated in CHO recombinant cells which express the human NK1 receptor. Membrane...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nM ΔG°: >-28.5kJ/molepH: 7.4 T: 2°CAssay Description:The affinity of compounds of the invention for the NK1 receptor was evaluated in CHO recombinant cells which express the human NK1 receptor. Membrane...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nM ΔG°: >-28.5kJ/molepH: 7.4 T: 2°CAssay Description:The affinity of compounds of the invention for the NK2 receptor was evaluated in CHO recombinant cells which express the human NK2 receptor. Membrane...More data for this Ligand-Target Pair
Affinity DataKi: 1.27E+4nM ΔG°: -27.9kJ/molepH: 7.4 T: 2°CAssay Description:The affinity of compounds of the invention for the NK1 receptor was evaluated in CHO recombinant cells which express the human NK1 receptor. Membrane...More data for this Ligand-Target Pair
Affinity DataKi: 1.40E+4nM ΔG°: -27.7kJ/molepH: 7.4 T: 2°CAssay Description:The affinity of compounds of the invention for the NK2 receptor was evaluated in CHO recombinant cells which express the human NK2 receptor. Membrane...More data for this Ligand-Target Pair
Affinity DataKi: 1.72E+4nM ΔG°: -27.2kJ/molepH: 7.4 T: 2°CAssay Description:The affinity of compounds of the invention for the NK1 receptor was evaluated in CHO recombinant cells which express the human NK1 receptor. Membrane...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Changes in intracellular calcium levels are a recognized indicator of G protein-coupled receptor activity. The efficacy of compounds of the invention...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Changes in intracellular calcium levels are a recognized indicator of G protein-coupled receptor activity. The efficacy of compounds of the invention...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Changes in intracellular calcium levels are a recognized indicator of G protein-coupled receptor activity. The efficacy of compounds of the invention...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Changes in intracellular calcium levels are a recognized indicator of G protein-coupled receptor activity. The efficacy of compounds of the invention...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Changes in intracellular calcium levels are a recognized indicator of G protein-coupled receptor activity. The efficacy of compounds of the invention...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Changes in intracellular calcium levels are a recognized indicator of G protein-coupled receptor activity. The efficacy of compounds of the invention...More data for this Ligand-Target Pair
Affinity DataIC50: 7nMAssay Description:Changes in intracellular calcium levels are a recognized indicator of G protein-coupled receptor activity. The efficacy of compounds of the invention...More data for this Ligand-Target Pair