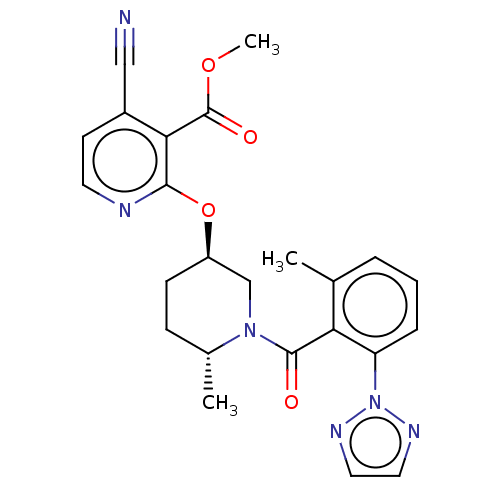
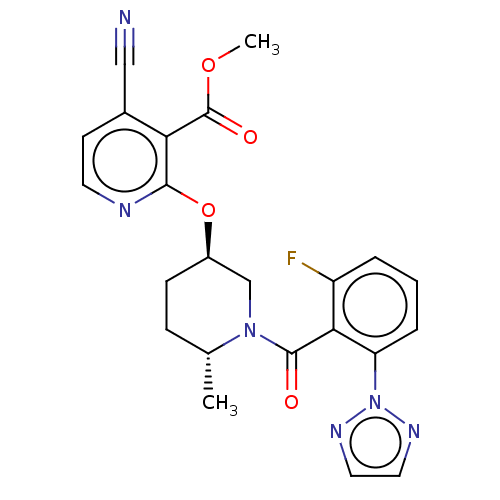
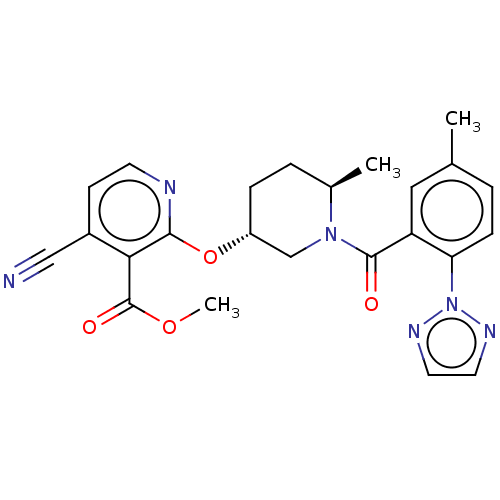
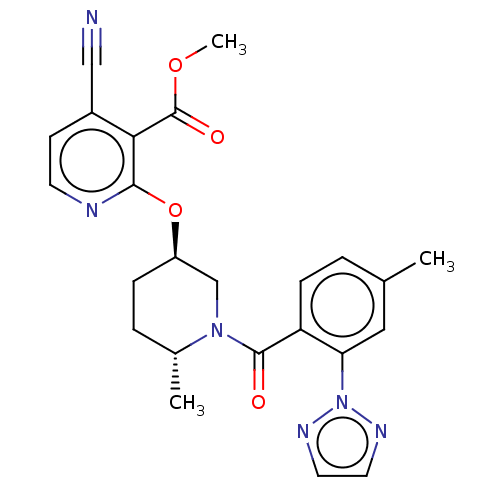
Affinity DataKi: 0.260nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 0.260nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 0.330nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 0.370nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 0.420nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 0.420nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 0.440nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 0.440nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 0.460nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 0.470nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 0.480nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 0.520nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 0.530nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 0.540nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 0.580nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 0.660nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 0.680nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 0.760nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 0.990nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 1.90nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 25.8nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 30.8nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 37.1nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 48nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 56nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 57.8nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 63nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 73.8nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 75.6nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 86.6nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 117nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 128nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 144nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 153nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 170nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 174nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 175nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 178nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 188nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 210nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 241nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 269nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair
Affinity DataKi: 332nMAssay Description:Described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430.More data for this Ligand-Target Pair