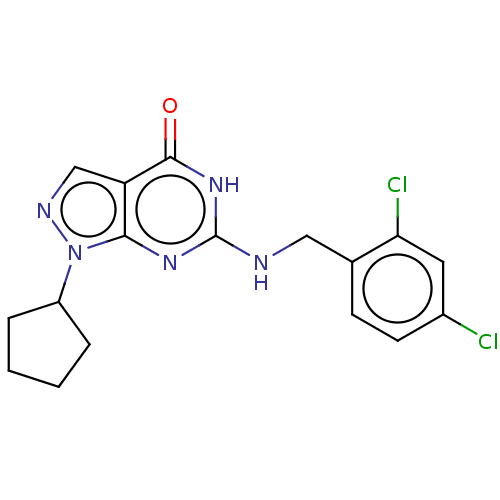
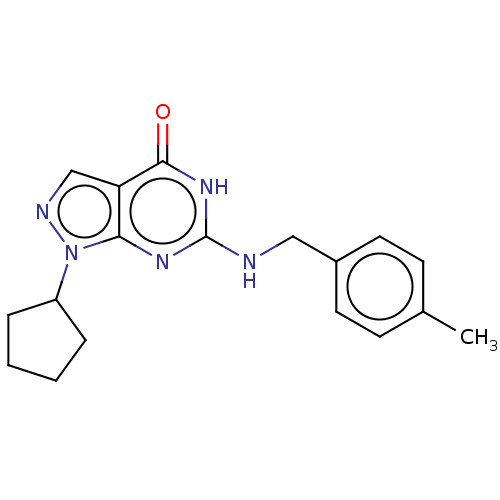
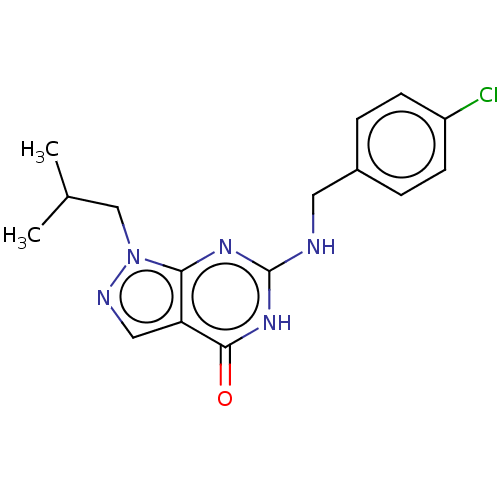
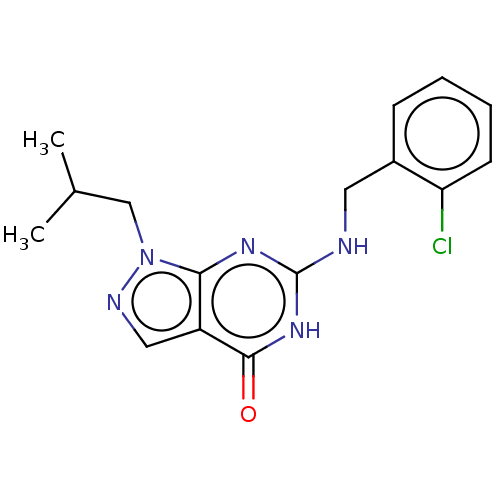
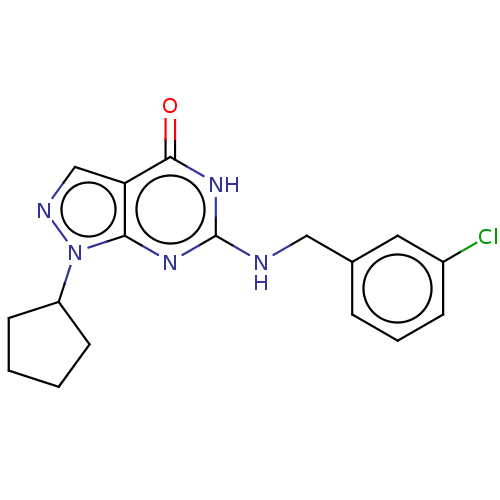
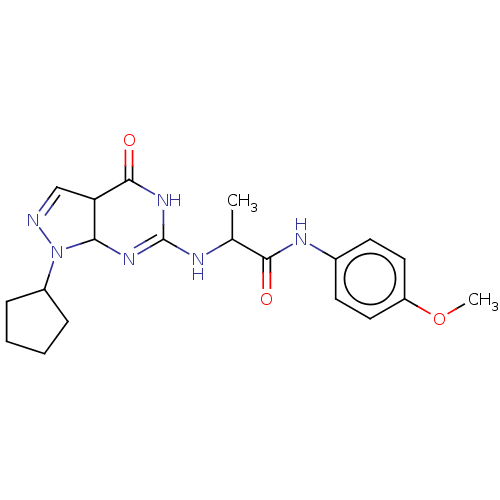
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 0.600nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
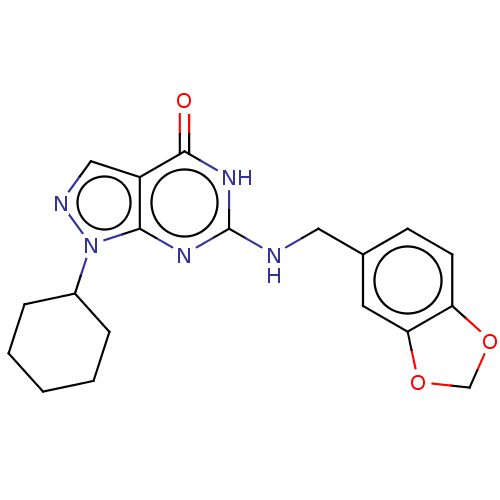
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 1nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
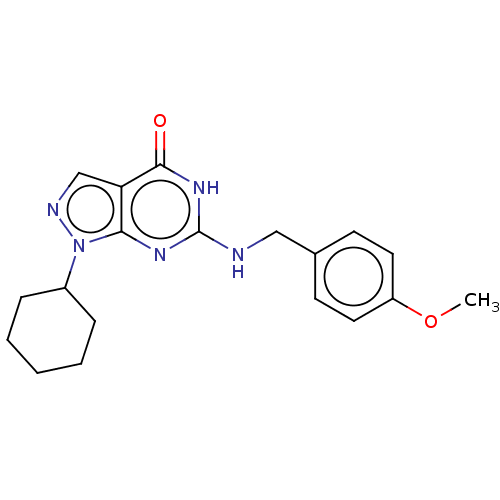
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 3nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
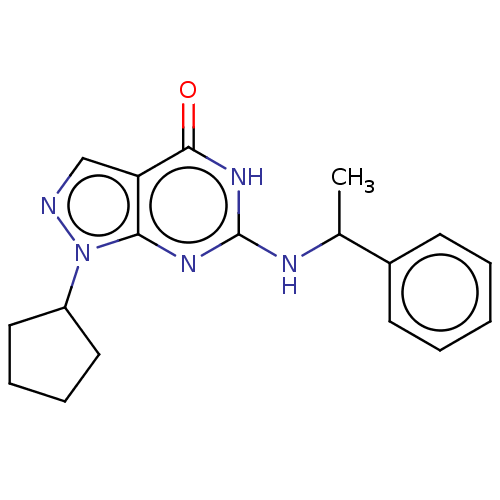
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 4nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 6nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 6nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 6nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 6nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 8nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 12nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 14nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 14nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 15nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 15nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 17nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 17nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 18nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 18nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 19nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 19nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 19nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 20nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 20nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 20nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 21nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 23nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 23nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 25nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 25nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 25nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 26nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 26nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 26nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 28nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 28nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 29nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 29nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 32nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 33nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 36nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 37nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 39nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 39nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 39nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 40nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 40nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 42nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 43nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 43nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair
TargetHigh affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A(Homo sapiens (Human))
Sun Yat-Sen University
US Patent
Sun Yat-Sen University
US Patent
Affinity DataIC50: 44nMAssay Description:Inhibition activities of all the N-substituted pyrazolo [3,4-d] pyrimidine ketone compounds according to the present invention to the phosphodiestera...More data for this Ligand-Target Pair






 3D Structure (crystal)
3D Structure (crystal)