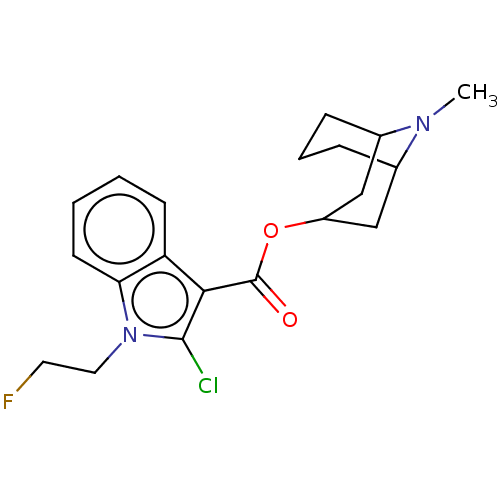
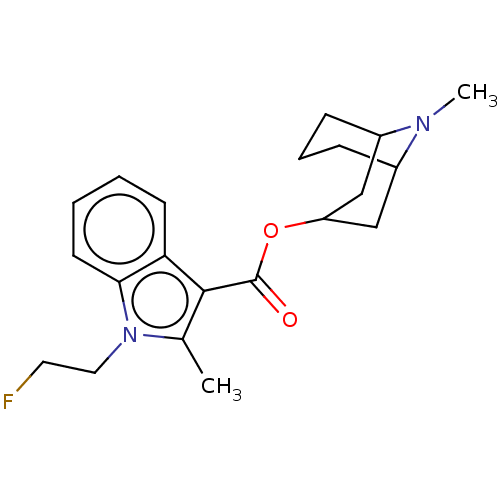
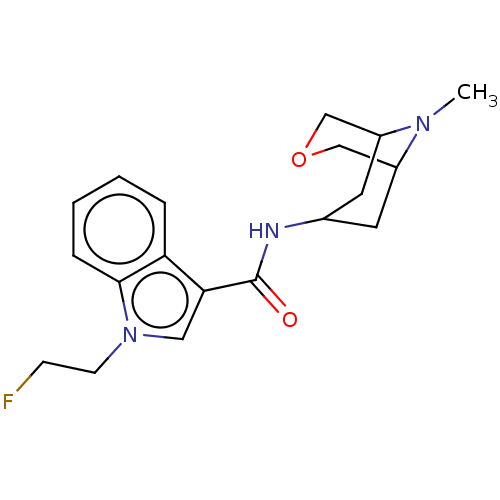
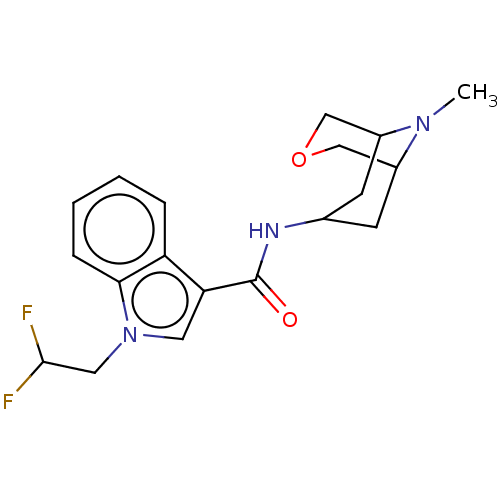
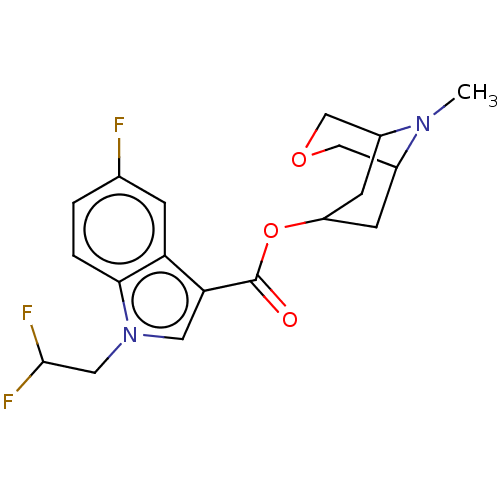
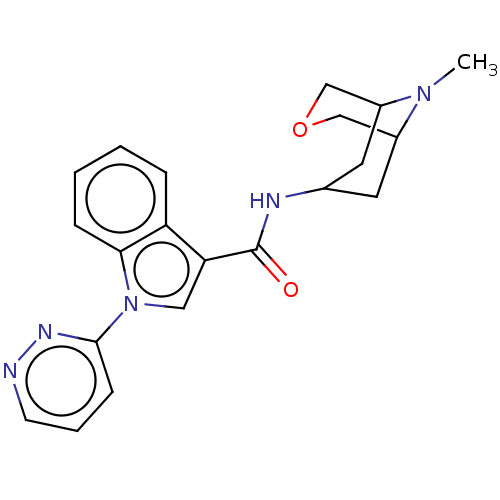
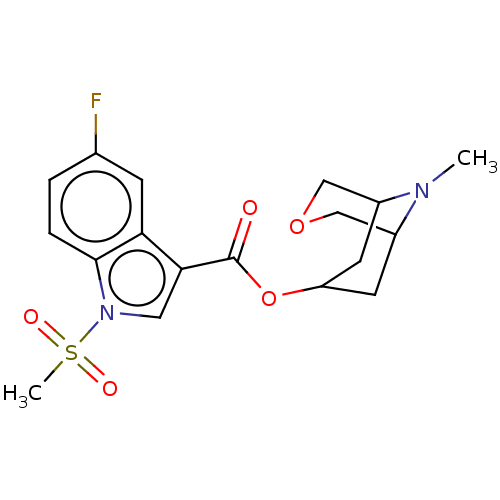
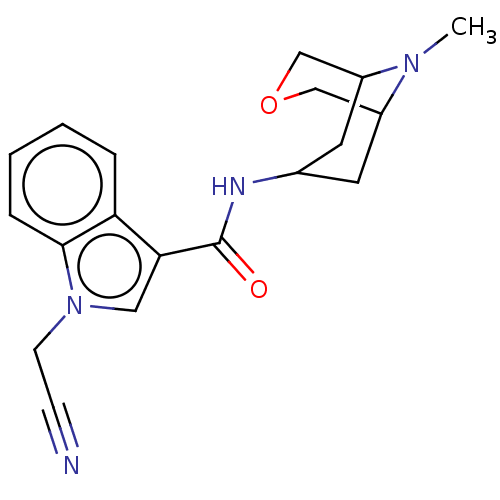
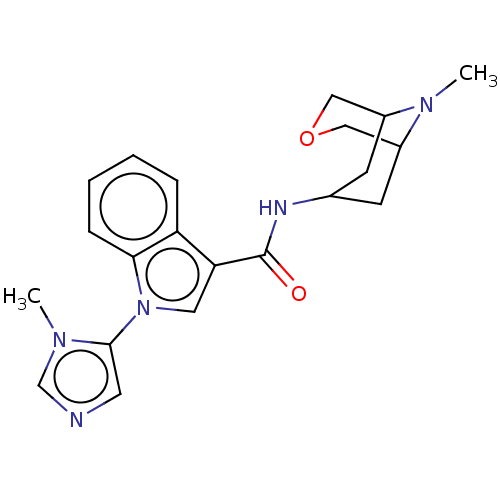
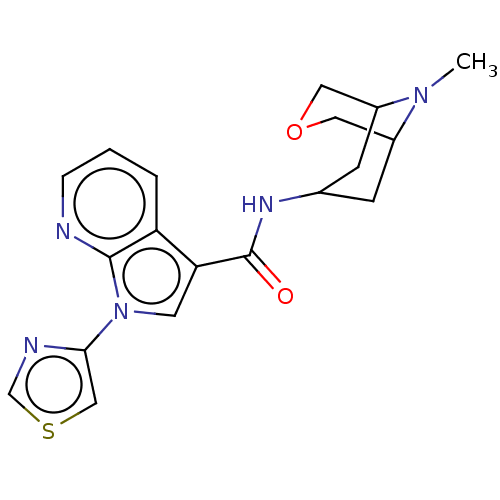
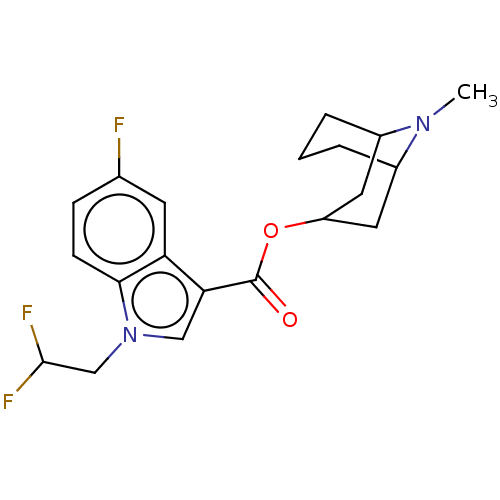
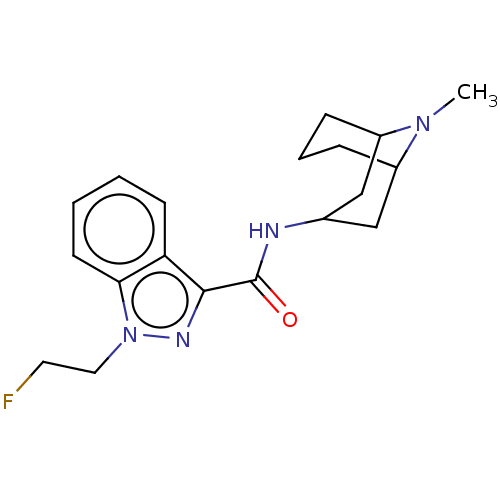
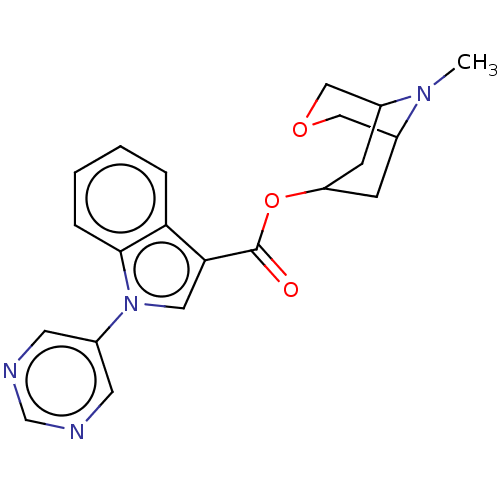
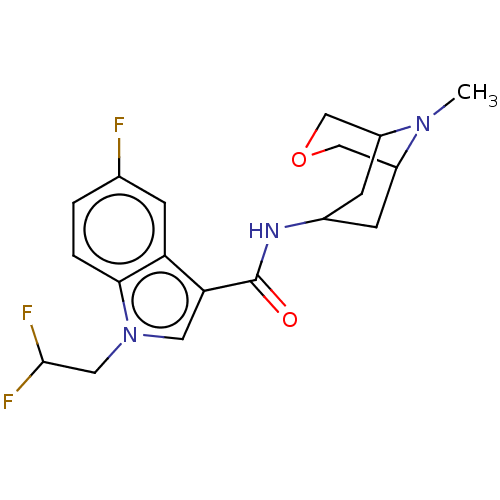
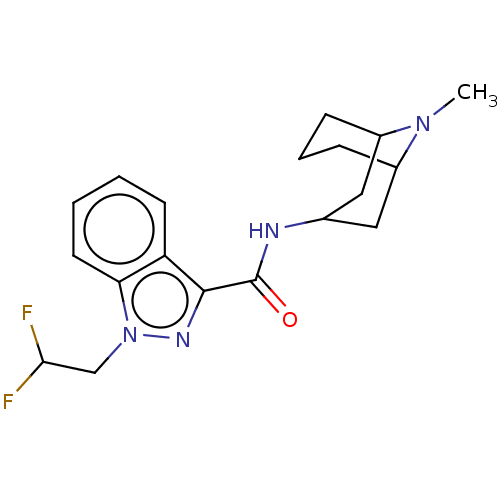
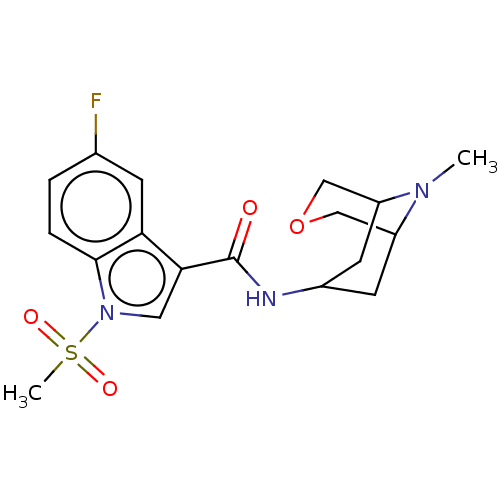
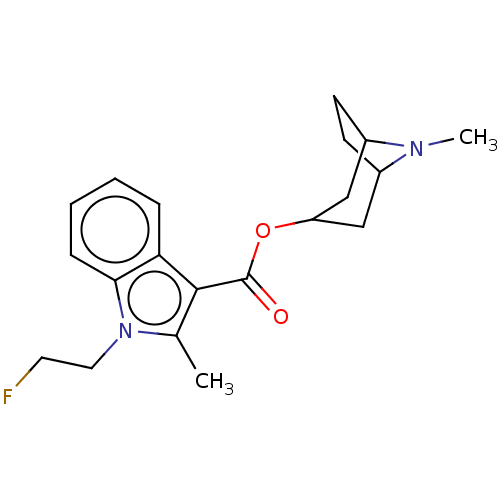
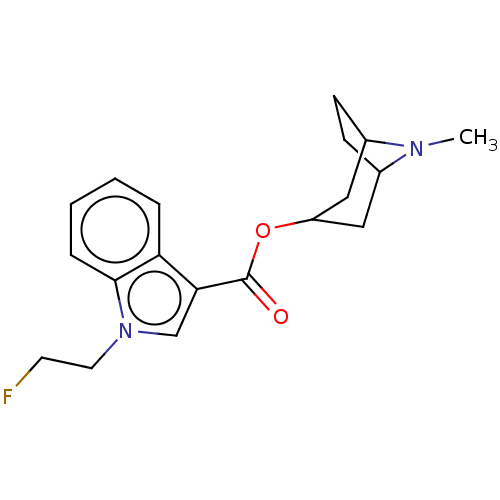
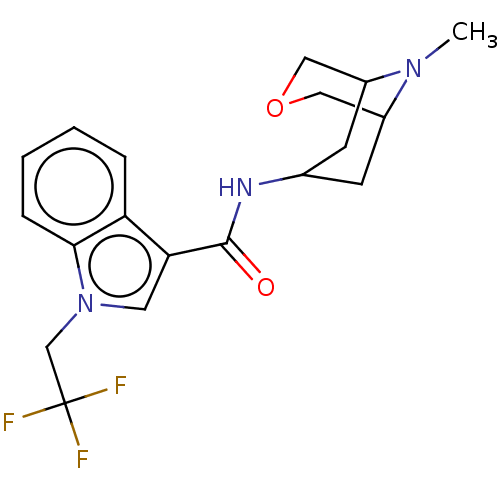
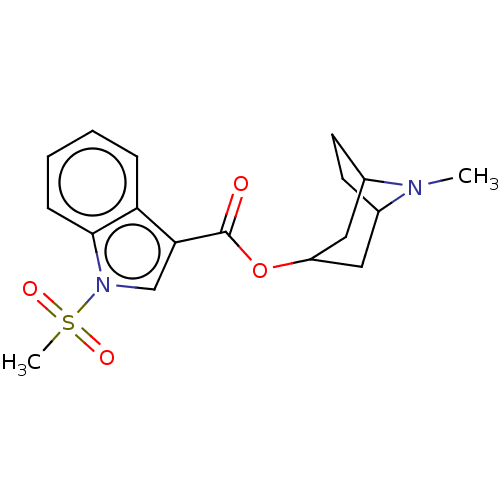
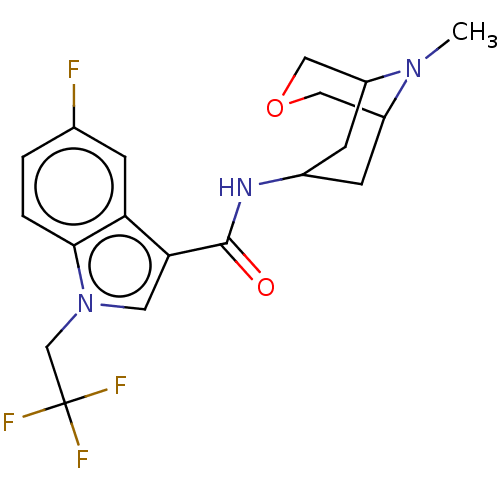
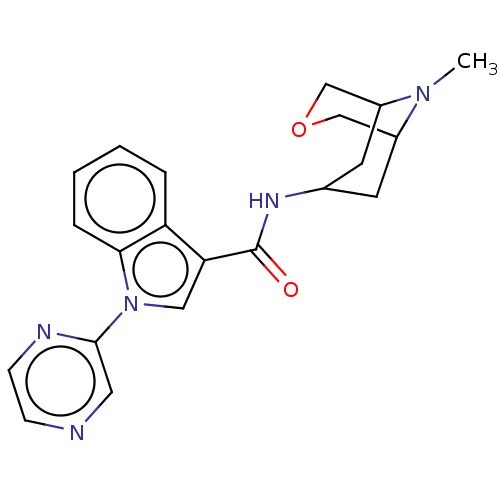
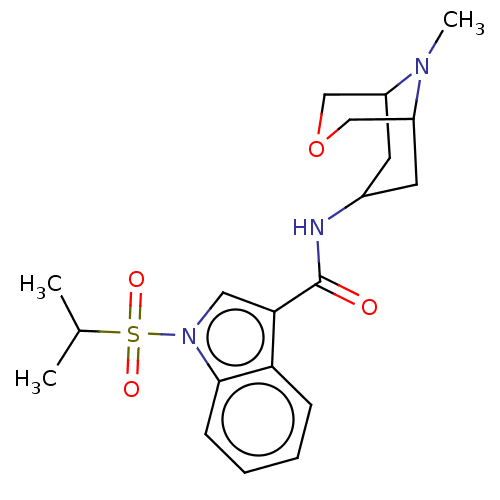
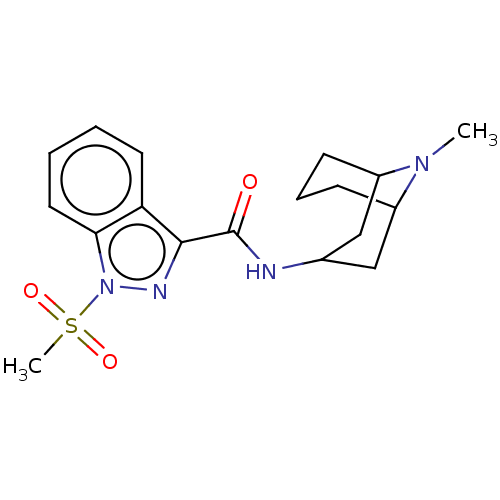
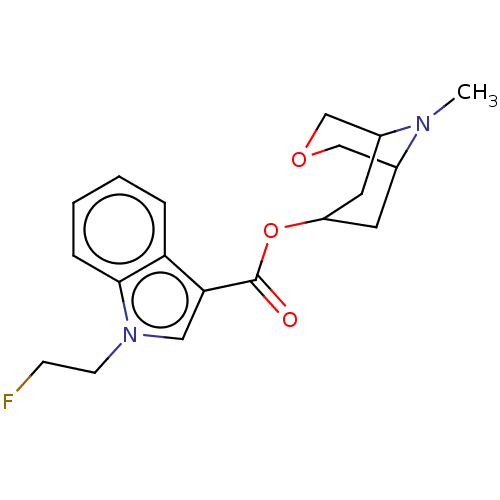
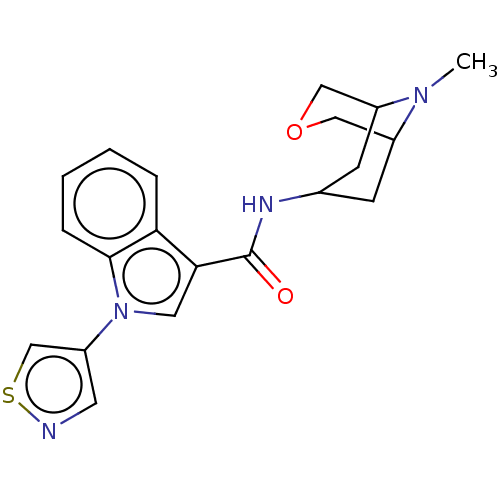
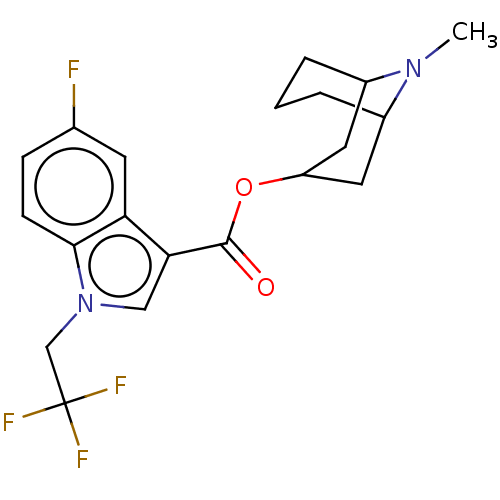
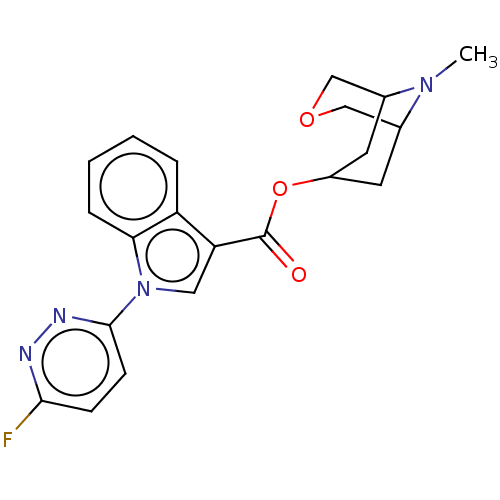
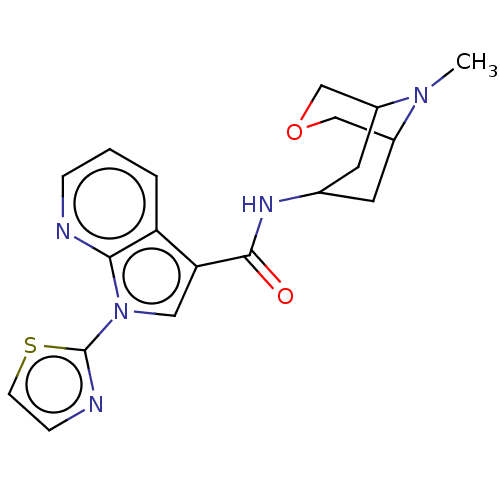
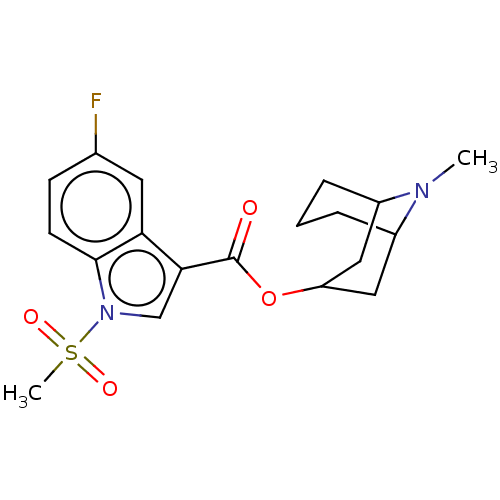
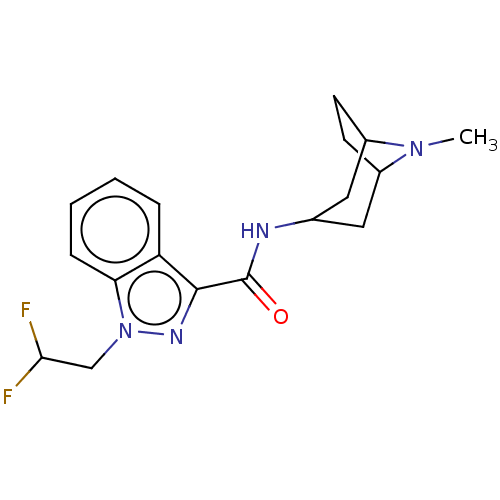
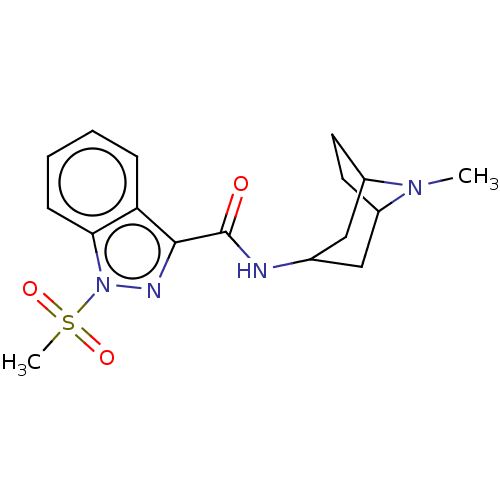
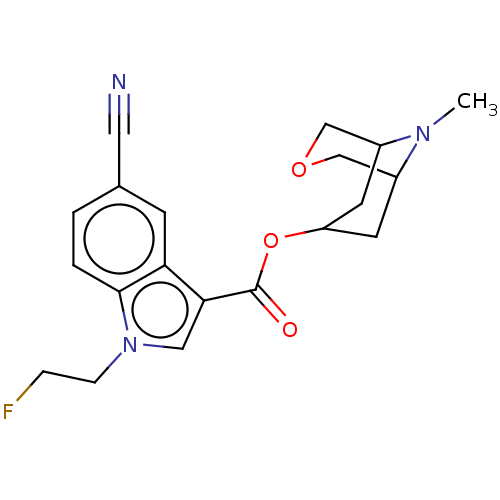
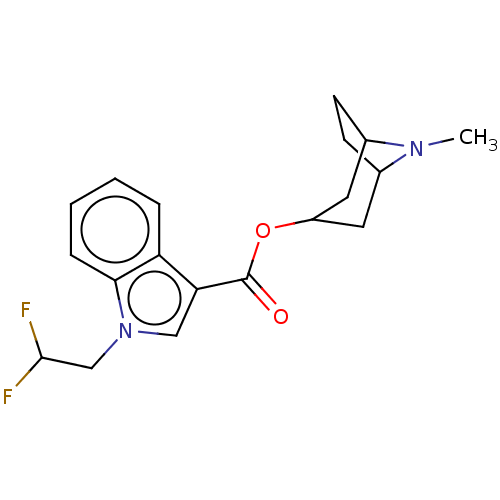
Affinity DataIC50: 0.180nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.220nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.220nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.230nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.240nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.280nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.290nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.330nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.330nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.390nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.390nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.410nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.420nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.420nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.440nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.450nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.460nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.490nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.490nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.530nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.530nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.530nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.540nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.540nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.550nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.570nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.590nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.610nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.640nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.660nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.680nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.680nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.740nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.75nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.75nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.75nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.75nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.770nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair
Affinity DataIC50: 0.780nMT: 2°CAssay Description:The 5HT3 antagonist activity of the compounds of the invention was determined by measuring the ability of the compounds to inhibit the calcium flux a...More data for this Ligand-Target Pair