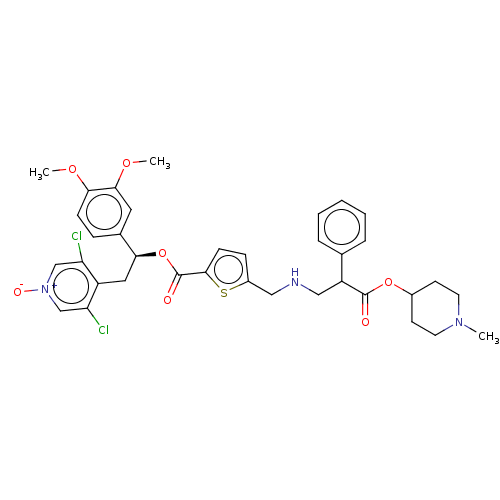
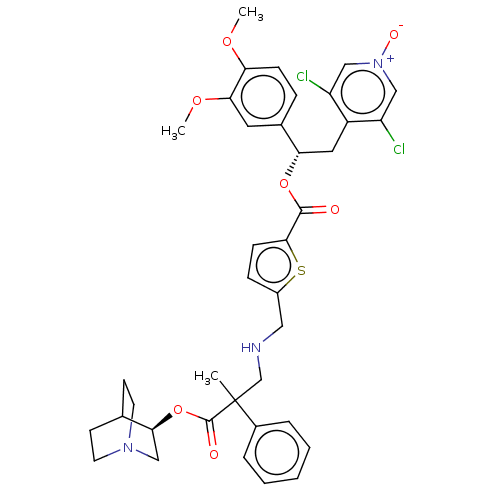
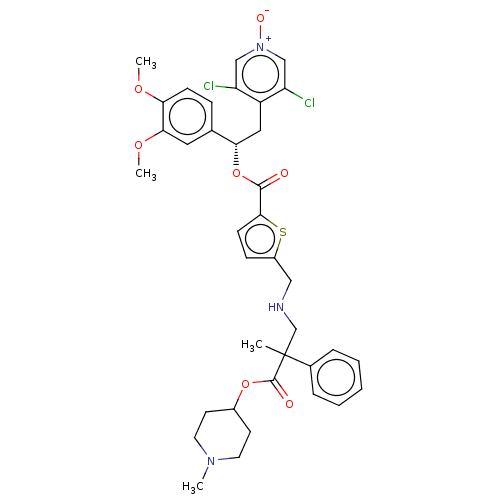
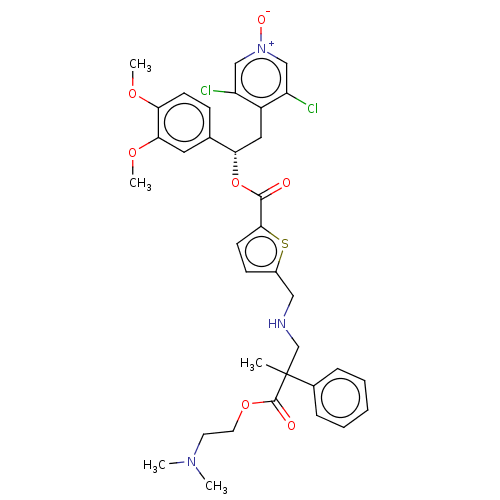
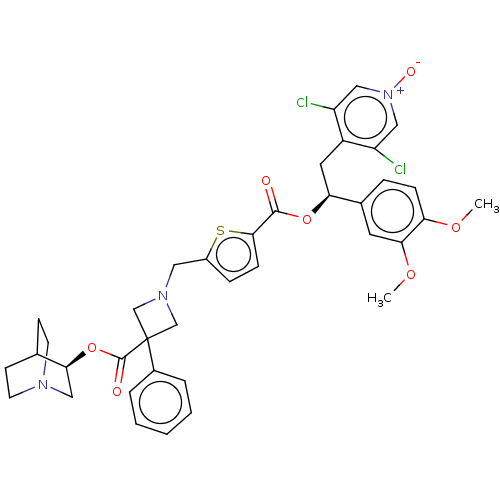
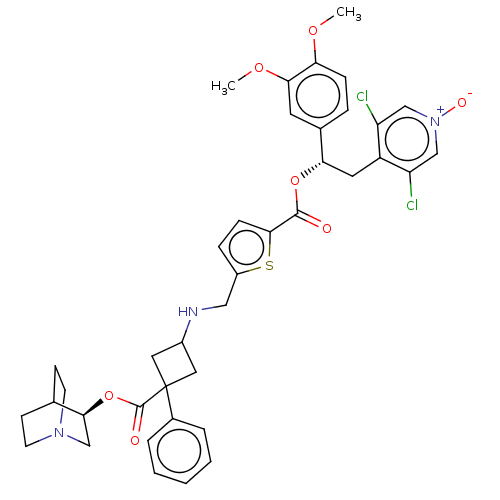
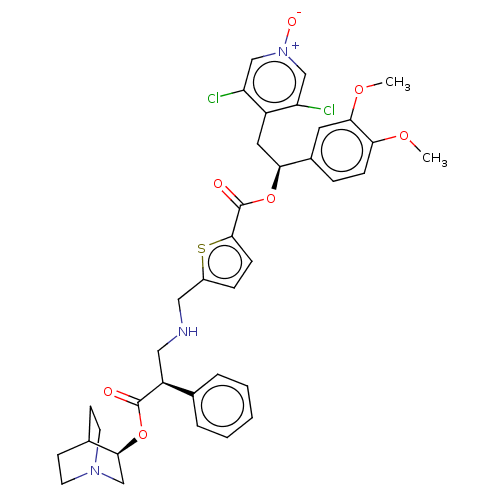
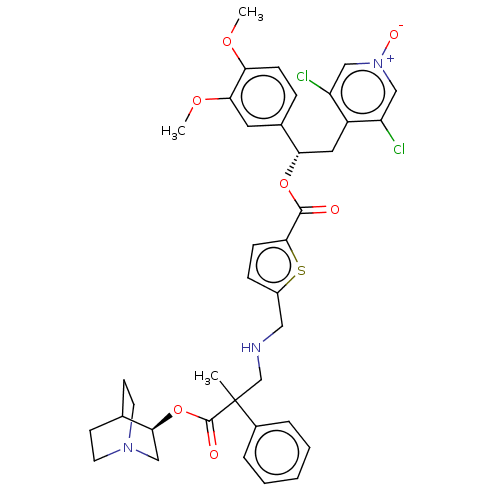
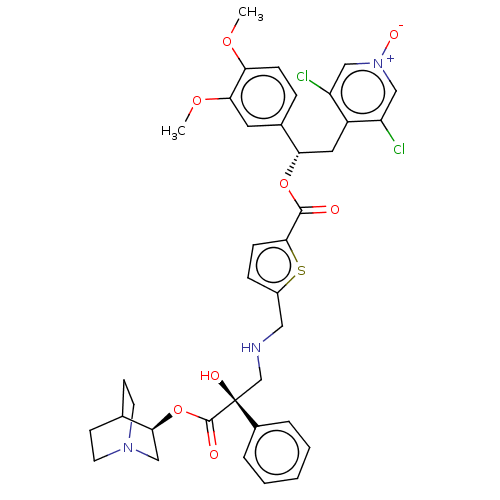
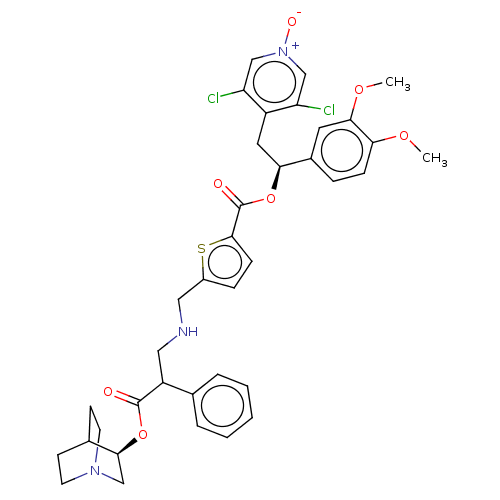
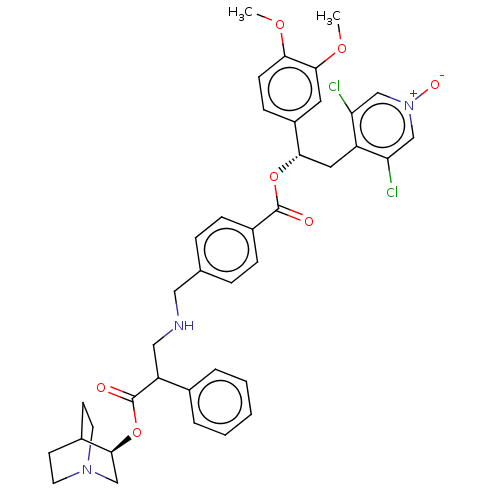
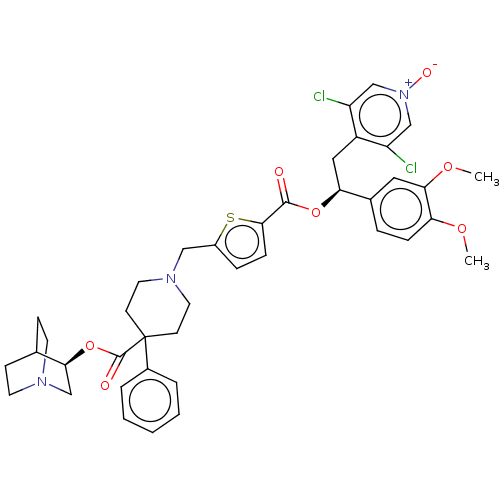
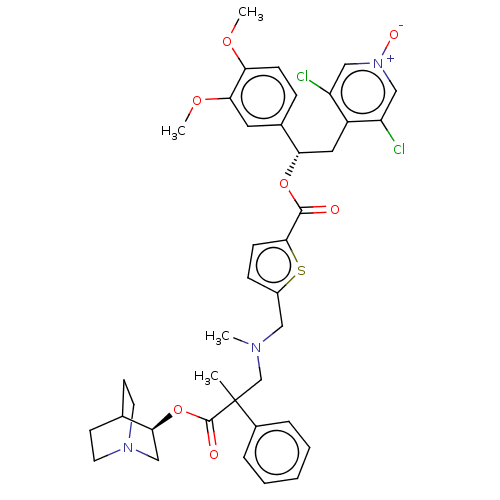
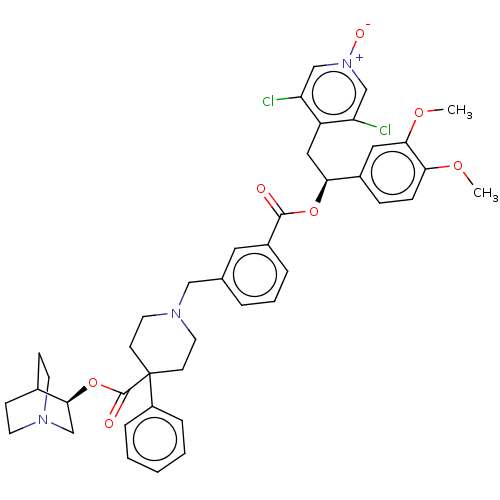
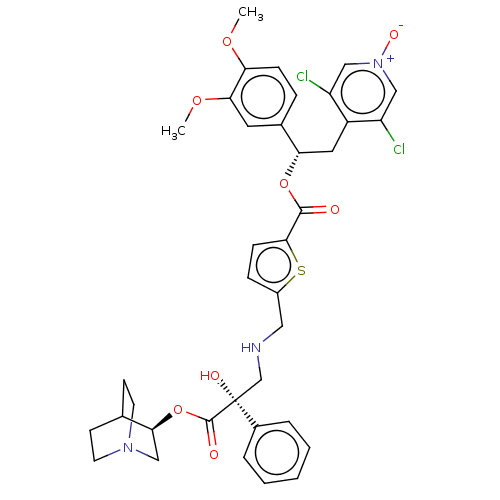
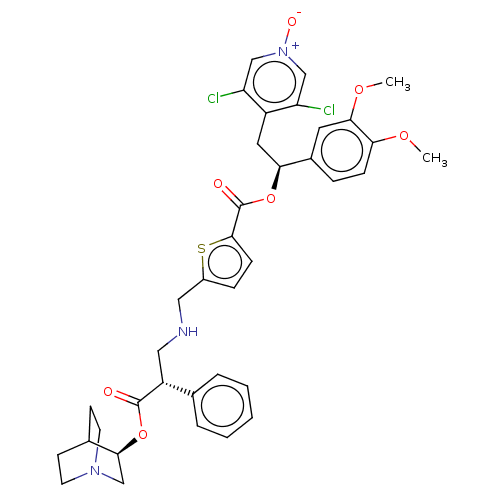
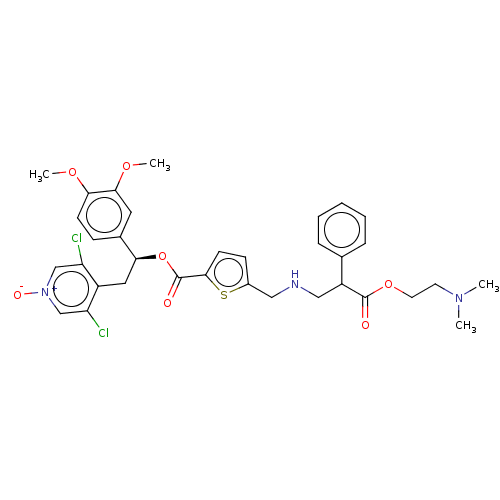
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: <1nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
Affinity DataIC50: <1nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: <1nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: <1nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
Affinity DataIC50: <1nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: <1nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
Affinity DataIC50: <1nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
Affinity DataIC50: <1nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: <1nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
Affinity DataIC50: <1nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: <1nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: <1nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: <1nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
Affinity DataIC50: <1nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair
TargetcAMP-specific 3',5'-cyclic phosphodiesterase 4B(Homo sapiens (Human))
Chiesi Farmaceutici
US Patent
Chiesi Farmaceutici
US Patent
Affinity DataIC50: 5.5nMAssay Description:PDE4B2 activity is detected using the LANCE Ultra cAMP homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay from Perkin E...More data for this Ligand-Target Pair
Affinity DataIC50: 5.5nMAssay Description:Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without...More data for this Ligand-Target Pair