Affinity DataKi: 140nM ΔG°: -39.8kJ/molepH: 7.0 T: 2°CAssay Description:A fluorescence assay was used to determine the kinetic constants for the oxidation of 3alpha-androstanediol. The fluorescence emission of NADPH at 45...More data for this Ligand-Target Pair
Affinity DataKi: 3.10E+3nM ΔG°: -32.0kJ/molepH: 7.0 T: 2°CAssay Description:Enzyme activity was measured in the reductive direction against varying concentrations of androsterone. Initial velocities were measured by observing...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of recombinant AKR1C3 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:Inhibition of AKR1C3 by fluorimetric methodMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 370nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 370nMAssay Description:Inhibition of recombinant AKR1C2 assessed as NADP+ dependent oxidation of S-tetralol by fluorescence assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 370nMAssay Description:Inhibition of AKR1C2 by fluorimetric methodMore data for this Ligand-Target Pair
Affinity DataIC50: 410nMAssay Description:Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1...More data for this Ligand-Target Pair
Affinity DataIC50: 410nMAssay Description:Inhibition of AKR1C3 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 440nMAssay Description:Inhibition of recombinant N-terminal GST-tagged human AKR1C3 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat...More data for this Ligand-Target Pair
Affinity DataIC50: 440nMAssay Description:Inhibition of type-1 human steroid 5-alpha-reductaseMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 530nMAssay Description:Inhibition of recombinant N-terminal GST-tagged human AKR1C2 expressed in Escherichia coli BL21 (DE) Codon Plus RP cells using S-tetralol as substrat...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 530nMAssay Description:Antagonistic activity against TP-receptor by inhibition of U 46619-induced contraction of isolated guinea pig tracheaMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 630nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 980nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.65E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 2.64E+3nMAssay Description:Inhibition of AKR1C1 (unknown origin)More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 2.64E+3nMAssay Description:Inhibition of human recombinant N-terminal His6-tagged AKR1C1 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 3.14E+3nMAssay Description:Inhibition of AKR1C2 (unknown origin)More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University Of Pennsylvania
US Patent
University Of Pennsylvania
US Patent
Affinity DataIC50: 3.14E+3nMAssay Description:Inhibition of human recombinant N-terminal His6-tagged AKR1C2 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1...More data for this Ligand-Target Pair
Affinity DataIC50: 8.63E+3nMAssay Description:Thromboxane (TXA2) receptor antagonist activity using human plateletMore data for this Ligand-Target Pair

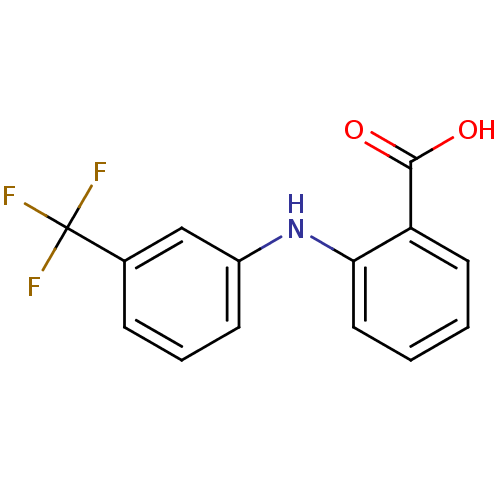
 3D Structure (crystal)
3D Structure (crystal)