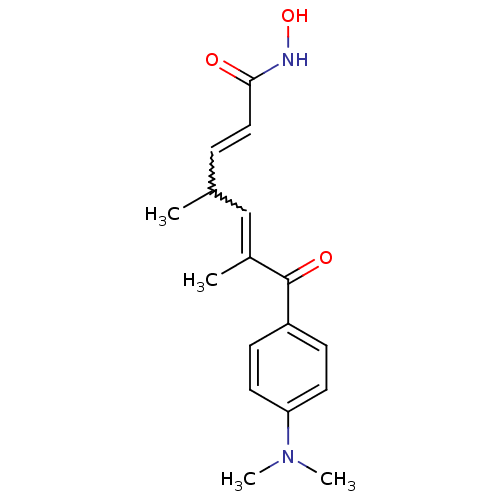
TargetHistone deacetylase 7(Homo sapiens (Human))
St. Jude Children'S Research Hospital
Curated by ChEMBL
St. Jude Children'S Research Hospital
Curated by ChEMBL
Affinity DataKi: 195nMAssay Description:Inhibition of human HDAC7More data for this Ligand-Target Pair
Affinity DataKi: 504nMAssay Description:Inhibition of HDAC in human Hela cells nuclear extracts by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of HDAC in human HeLa cell nuclear extract using BML-KI104 Fluor de Lys as substrate by fluorescence-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of HDAC in human HeLa cells using Ac-Arg-Gly-Lys(Ac)-AMC as fluorescent substrate after 20 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40nMAssay Description:Inhibitory activity against Histone deacetylase (HDAC) in K 562 erythroleukemia cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.60nMAssay Description:Inhibitory activity against partially purified histone deacetylase of human leukemia K562 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of histone deacetylase (HDAC) activity in HeLa cell nuclear extractMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of HDAC in human HeLa cell nuclear extracts after 15 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human HDAC in HeLa cells by flour de lys assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.5nMAssay Description:Inhibition of HDAC in human HeLa cell extract after 15 mins by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of human HDAC after 30 minsMore data for this Ligand-Target Pair
TargetHistone deacetylase 7(Homo sapiens (Human))
St. Jude Children'S Research Hospital
Curated by ChEMBL
St. Jude Children'S Research Hospital
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:Inhibition of human HDAC7 by fluorimetric assayMore data for this Ligand-Target Pair
TargetHistone deacetylase 7(Homo sapiens (Human))
St. Jude Children'S Research Hospital
Curated by ChEMBL
St. Jude Children'S Research Hospital
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:Inhibition of human HDAC7More data for this Ligand-Target Pair
TargetHistone deacetylase 7(Homo sapiens (Human))
St. Jude Children'S Research Hospital
Curated by ChEMBL
St. Jude Children'S Research Hospital
Curated by ChEMBL
Affinity DataIC50: 23nMAssay Description:Inhibition of human HDAC7 using fluorogenic tetrapeptide RHKKAc as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Concentration required to inhibit human Histone deacetylase (HDAC) enzyme by 50%More data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Inhibitory activity against histone deacetylase (HDAC) enzyme obtained from H1299 human lung carcinoma cell lysatesMore data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Inhibition of human HDAC in human HeLa cell nuclear extract after 15 mins by colorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 53nMAssay Description:Inhibitory activity against Histone deacetylase (HDAC) from SNU-16 (human gastric adenocarcinoma) cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Inhibitory concentration against human histone deacetylase (HDAC) from HeLa cells with substrate 5aMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Concentration required to inhibit the partially purified HDAC enzyme by 50% obtained from H1299 cell lysateMore data for this Ligand-Target Pair
TargetHistone deacetylase 7(Homo sapiens (Human))
St. Jude Children'S Research Hospital
Curated by ChEMBL
St. Jude Children'S Research Hospital
Curated by ChEMBL
Affinity DataIC50: 139nMAssay Description:Inhibition of human recombinant HDAC7 using fluorophore-conjugated substrate Boc-L-Lys(Ac)-AMC after 60 minsMore data for this Ligand-Target Pair
TargetHistone deacetylase 7(Homo sapiens (Human))
St. Jude Children'S Research Hospital
Curated by ChEMBL
St. Jude Children'S Research Hospital
Curated by ChEMBL
Affinity DataIC50: 197nMAssay Description:HDAC enzyme inhibition assays were performed using purified HDACs 1-10 essentially as described in Beckers et al., 2007, Int. J. Cancer., 121:1138-48...More data for this Ligand-Target Pair
TargetHistone deacetylase 7(Homo sapiens (Human))
St. Jude Children'S Research Hospital
Curated by ChEMBL
St. Jude Children'S Research Hospital
Curated by ChEMBL
Affinity DataIC50: 200nMAssay Description:Inhibition of HDAC7 by in vitro deacetylation assayMore data for this Ligand-Target Pair
TargetHistone deacetylase 7(Homo sapiens (Human))
St. Jude Children'S Research Hospital
Curated by ChEMBL
St. Jude Children'S Research Hospital
Curated by ChEMBL
Affinity DataIC50: 663nMpH: 7.5Assay Description:Biochemical assays of HDAC activity were carried out by Nanosyn in a reaction volume of 10 ul in 384-well microplates. A standard enzymatic reaction ...More data for this Ligand-Target Pair
TargetHistone deacetylase 7(Homo sapiens (Human))
St. Jude Children'S Research Hospital
Curated by ChEMBL
St. Jude Children'S Research Hospital
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of HDAC7 (unknown origin) using Boc-Lys(trifluoroacetyl)-AMC fluorogenic acetylated peptide substrate by fluorometric assayMore data for this Ligand-Target Pair
TargetHistone deacetylase 7(Homo sapiens (Human))
St. Jude Children'S Research Hospital
Curated by ChEMBL
St. Jude Children'S Research Hospital
Curated by ChEMBL
Affinity DataIC50: 2.95E+3nMpH: 8.0Assay Description:Briefly, recombinant HDACs and substrates were diluted in reaction buffer (50 mM Tris-HCl, pH 8.0, 137 mM NaCl, 2.7mM KCl, 1mM MgCl2, 1 mg/ml BSA, 1%...More data for this Ligand-Target Pair
Affinity DataIC50: 4.29E+3nMAssay Description: I. Compound handling: Testing compounds were dissolved in 100% DMSO to a specific concentration. The serial dilution was conducted by epMotion 5070 ...More data for this Ligand-Target Pair

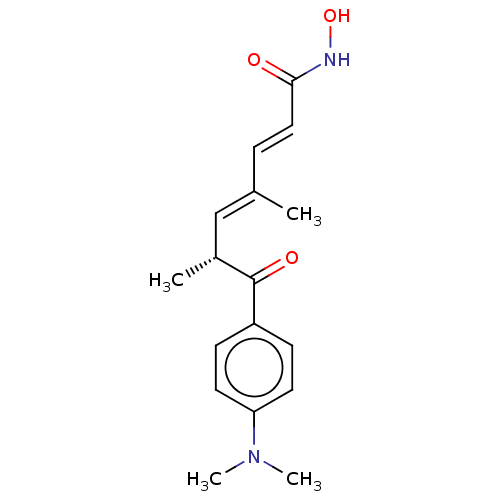
 3D Structure (crystal)
3D Structure (crystal)