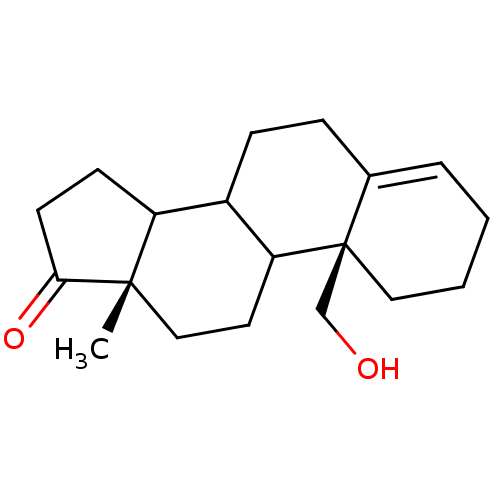
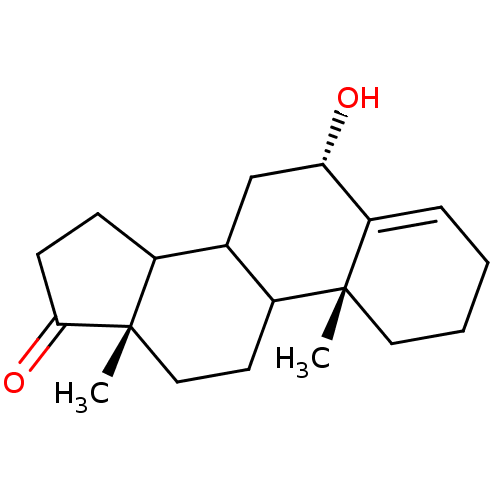
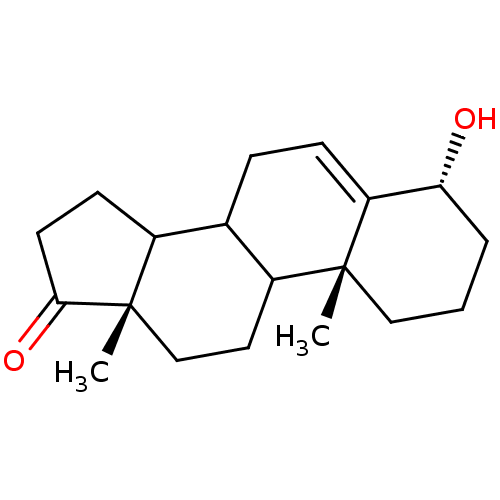
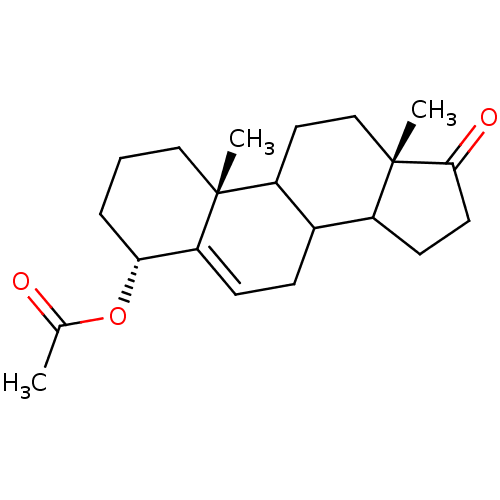
Affinity DataKi: 3.40nM ΔG°: -50.3kJ/mole IC50: 31nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 5.80nM ΔG°: -48.9kJ/mole IC50: 49nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 6nM ΔG°: -48.8kJ/mole IC50: 50nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 6.80nM ΔG°: -48.5kJ/mole IC50: 60nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 21nM ΔG°: -45.6kJ/mole IC50: 190nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 25nM ΔG°: -45.1kJ/mole IC50: 280nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 58nM ΔG°: -43.0kJ/mole IC50: 860nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 60nM ΔG°: -42.9kJ/mole IC50: 700nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 90nM ΔG°: -41.8kJ/mole IC50: 1.00E+3nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 110nM ΔG°: -41.3kJ/mole IC50: 940nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 120nM ΔG°: -41.1kJ/mole IC50: 660nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 250nM ΔG°: -39.2kJ/mole IC50: 1.40E+3nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 800nM ΔG°: -36.2kJ/mole IC50: 6.80E+3nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nM ΔG°: -35.6kJ/mole IC50: 6.90E+3nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+5nMpH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair
Affinity DatapH: 7.5 T: 2°CAssay Description:The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re...More data for this Ligand-Target Pair