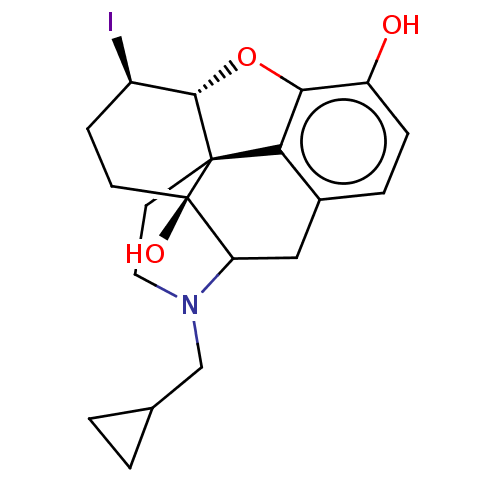
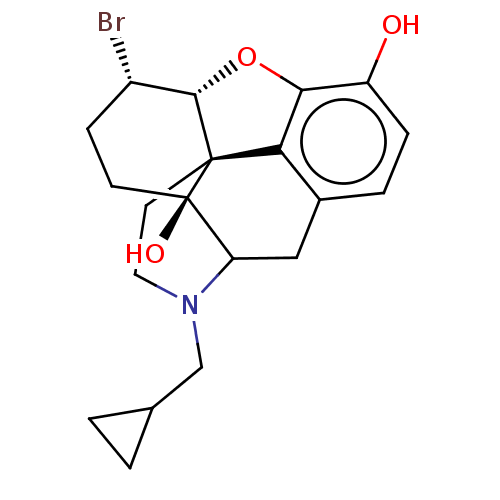
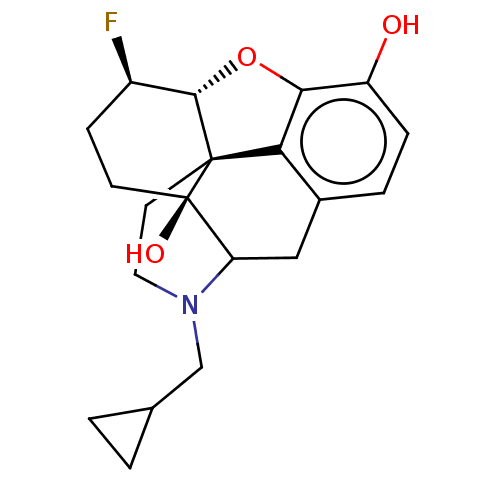
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 0.290nMAssay Description:In vito concentration required to displace [3H]DAGO (Kd = 0.7 nM and concentration is 1.7 nM) from opioid receptor mu in rat brain membranes.More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 0.320nMAssay Description:In vito concentration required to displace 9 (Kd = 1.6 nM and concentration is 1.8 nM) from opioid receptor kappa 1 in guinea brain membranes.More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 0.420nMAssay Description:In vito concentration required to displace 9 (Kd = 1.6 nM and concentration is 1.8 nM) from opioid receptor kappa 1 in guinea brain membranes.More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:In vito concentration required to displace [3H]cyclofoxy (Kd = 0.8 nM and concentration is 1.3 nM) from mu and kappa2 receptor in rat brain membranes...More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:In vito concentration required to displace 9 (Kd = 1.6 nM and concentration is 1.8 nM) from opioid receptor kappa 1 in guinea brain membranes.More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:In vito concentration required to displace [3H]DAGO (Kd = 0.7 nM and concentration is 1.7 nM) from opioid receptor mu in rat brain membranes.More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:In vito concentration required to displace 9 (Kd = 1.6 nM and concentration is 1.8 nM) from opioid receptor kappa 1 in guinea brain membranes.More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:In vito concentration required to displace [3H]BRM (Kd = 1.0 nM and concentration is 1.8 nM) from opioid receptor kappa 2 in guinea brain membranes.More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:In vito concentration required to displace [3H]DAGO (Kd = 0.7 nM and concentration is 1.7 nM) from opioid receptor mu in rat brain membranes.More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:In vito concentration required to displace [3H]DADLE (Kd = 1.6 nM and concentration is 1.9 nM) from high affinity delta-site in rat brain membranes.More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 2.10nMAssay Description:In vito concentration required to displace [3H]DADLE (Kd = 12.2 nM and concentration is 2.1 nM) from low affinity delta-site in rat brain membranes.More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:In vito concentration required to displace 9 (Kd = 1.6 nM and concentration is 1.8 nM) from opioid receptor kappa 1 in guinea brain membranes.More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 2.60nMAssay Description:In vito concentration required to displace [3H]cyclofoxy (Kd = 0.8 nM and concentration is 1.3 nM) from mu and kappa2 receptor in rat brain membranes...More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 2.70nMAssay Description:In vito concentration required to displace 9 (Kd = 1.6 nM and concentration is 1.8 nM) from opioid receptor kappa 2 in guinea brain membranes.More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 2.80nMAssay Description:In vito concentration required to displace 9 (Kd = 1.6 nM and concentration is 1.8 nM) from opioid receptor kappa 1 in guinea brain membranes.More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 3.30nMAssay Description:In vito concentration required to displace [3H]DADLE (Kd = 1.6 nM and concentration is 1.9 nM) from high affinity delta-site in rat brain membranes.More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 3.40nMAssay Description:In vito concentration required to displace [3H]DAGO (Kd = 0.7 nM and concentration is 1.7 nM) from opioid receptor mu in rat brain membranes.More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 3.70nMAssay Description:In vito concentration required to displace [3H]DAGO (Kd = 0.7 nM and concentration is 1.7 nM) from opioid receptor mu in rat brain membranes.More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 4.80nMAssay Description:In vito concentration required to displace [3H]DADLE (Kd = 12.2 nM and concentration is 2.1 nM) from low affinity delta-site in rat brain membranes.More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 4.90nMAssay Description:In vito concentration required to displace [3H]DADLE (Kd = 12.2 nM and concentration is 2.1 nM) from low affinity delta-site in rat brain membranes.More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 6.90nMAssay Description:In vito concentration required to displace [3H]DADLE (Kd = 12.2 nM and concentration is 2.1 nM) from low affinity delta-site in rat brain membranes.More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 7.20nMAssay Description:In vito concentration required to displace [3H]BRM (Kd = 1.0 nM and concentration is 1.8 nM) from opioid receptor kappa 2 in guinea brain membranes.More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 8.5nMAssay Description:In vito concentration required to displace [3H]cyclofoxy (Kd = 0.8 nM and concentration is 1.3 nM) from mu and kappa2 receptor in rat brain membranes...More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:In vito concentration required to displace [3H]DADLE (Kd = 1.6 nM and concentration is 1.9 nM) from high affinity delta-site in rat brain membranes.More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:In vito concentration required to displace [3H]DADLE (Kd = 12.2 nM and concentration is 2.1 nM) from low affinity delta-site in rat brain membranes.More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 17nMAssay Description:In vito concentration required to displace 9 (Kd = 1.6 nM and concentration is 1.8 nM) from opioid receptor kappa 1 in guinea brain membranes.More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 24nMAssay Description:In vito concentration required to displace 9 (Kd = 1.6 nM and concentration is 1.8 nM) from opioid receptor kappa 1 in guinea brain membranes.More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 46nMAssay Description:In vito concentration required to displace [3H]DADLE (Kd = 1.6 nM and concentration is 1.9 nM) from high affinity delta-site in rat brain membranes.More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 101nMAssay Description:In vito concentration required to displace [3H]-DADLE (Kd = 12.2 nM and concentration is 2.1 nM) from low affinity delta-site in rat brain membranes.More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 122nMAssay Description:In vito concentration required to displace [3H]DADLE (Kd = 1.6 nM and concentration is 1.9 nM) from high affinity delta-site in rat brain membranes.More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:In vito concentration required to displace 9 (Kd = 1.6 nM and concentration is 1.8 nM) from opioid receptor kappa 1 in guinea brain membranes.More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 0.770nMAssay Description:In vito concentration required to displace [3H]DAGO (Kd = 0.7 nM and concentration is 1.7 nM) from opioid receptor mu in rat brain membranes.More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 0.890nMAssay Description:In vito concentration required to displace [3H]cyclofoxy (Kd = 0.8 nM and concentration is 1.3 nM) from mu and kappa2 receptor in rat brain membranes...More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 2.60nMAssay Description:In vito concentration required to displace [3H]DAGO (Kd = 0.7 nM and concentration is 1.7 nM) from opioid receptor mu in rat brain membranes.More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 2.70nMAssay Description:In vito concentration required to displace [3H]DAGO (Kd = 0.7 nM and concentration is 1.7 nM) from opioid receptor mu in rat brain membranes.More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 3.10nMAssay Description:In vito concentration required to displace [3H]-DADLE (Kd = 12.2 nM and concentration is 2.1 nM) from low affinity delta-site in rat brain membranes.More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:In vito concentration required to displace [3H]-DADLE (Kd = 12.2 nM and concentration is 2.1 nM) from low affinity delta-site in rat brain membranes.More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:In vito concentration required to displace [3H]BRM (Kd = 1.0 nM and concentration is 1.8 nM) from opioid receptor kappa 2 in guinea brain membranes.More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 4.20nMAssay Description:In vito concentration required to displace [3H]DAGO (Kd = 0.7 nM and concentration is 1.7 nM) from opioid receptor mu in rat brain membranes.More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 4.30nMAssay Description:In vito concentration required to displace [3H]-BRM (Kd = 1.0 nM and concentration is 1.8 nM) from opioid receptor kappa 2 in guinea brain membranes.More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 4.30nMAssay Description:In vito concentration required to displace [3H]DADLE (Kd = 1.6 nM and concentration is 1.9 nM) from high affinity delta-site in rat brain membranes.More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 5.60nMAssay Description:In vito concentration required to displace [3H]cyclofoxy (Kd = 0.8 nM and concentration is 1.3 nM) from mu and kappa2 receptor in rat brain membranes...More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 5.80nMAssay Description:In vito concentration required to displace [3H]DADLE (Kd = 12.2 nM and concentration is 2.1 nM) from low affinity delta-site in rat brain membranes.More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:In vito concentration required to displace 9 (Kd = 1.6 nM and concentration is 1.8 nM) from opioid receptor kappa 1 in guinea brain membranes.More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 6.80nMAssay Description:In vito concentration required to displace [3H]DADLE (Kd = 12.2 nM and concentration is 2.1 nM) from low affinity delta-site in rat brain membranes.More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 7.20nMAssay Description:In vito concentration required to displace [3H]-DADLE (Kd = 12.2 nM and concentration is 2.1 nM) from low affinity delta-site in rat brain membranes.More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 7.70nMAssay Description:In vito concentration required to displace [3H]BRM (Kd = 1.0 nM and concentration is 1.8 nM) from opioid receptor kappa 2 in guinea brain membranes.More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 7.90nMAssay Description:In vito concentration required to displace [3H]BRM (Kd = 1.0 nM and concentration is 1.8 nM) from opioid receptor kappa 2 in guinea brain membranes.More data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 8.10nMAssay Description:In vito concentration required to displace [3H]DADLE (Kd = 12.2 nM and concentration is 2.1 nM) from low affinity delta-site in rat brain membranes.More data for this Ligand-Target Pair
TargetMu-type opioid receptor(Rattus norvegicus (rat))
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
National Institute Of Diabetes And Digestive And Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:In vito concentration required to displace [3H]DADLE (Kd = 1.6 nM and concentration is 1.9 nM) from high affinity delta-site in rat brain membranes.More data for this Ligand-Target Pair