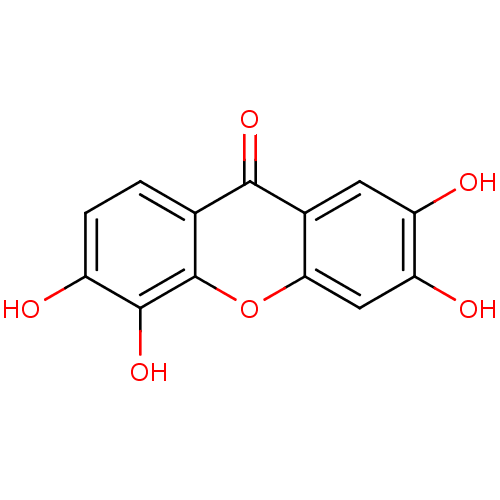
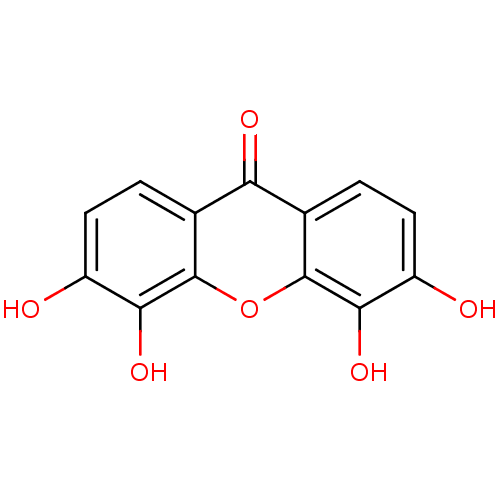
Affinity DataKi: 1.42E+4nMAssay Description:Inhibition of ACE by Lineweaver-Burk plotMore data for this Ligand-Target Pair
Affinity DataKi: 3.42E+4nMAssay Description:Inhibition of ACE by Lineweaver-Burk plotMore data for this Ligand-Target Pair
Affinity DataKi: 1.26E+5nMAssay Description:Inhibition of ACE by Lineweaver-Burk plotMore data for this Ligand-Target Pair
Affinity DataKi: 2.50E+5nMAssay Description:Inhibition of ACE by Lineweaver-Burk plotMore data for this Ligand-Target Pair
Affinity DataIC50: 3.54E+4nMAssay Description:Inhibition of ACEMore data for this Ligand-Target Pair
Affinity DataIC50: 6.92E+4nMAssay Description:Inhibition of ACEMore data for this Ligand-Target Pair
Affinity DataIC50: 2.39E+5nMAssay Description:Inhibition of ACEMore data for this Ligand-Target Pair
Affinity DataIC50: 5.31E+5nMAssay Description:Inhibition of ACEMore data for this Ligand-Target Pair
Affinity DataIC50: 7.69E+5nMAssay Description:Inhibition of ACEMore data for this Ligand-Target Pair