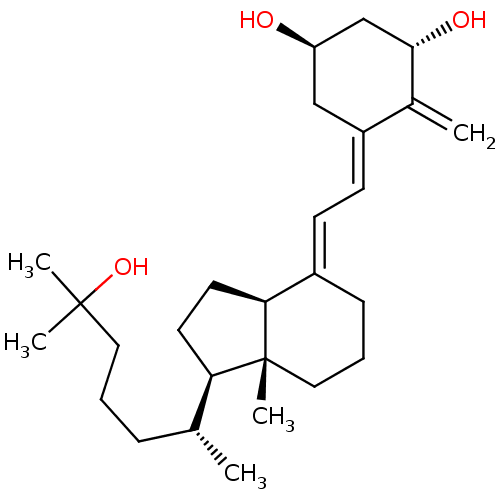
Affinity DataIC50: 0.700nMAssay Description:Displacement of [3H]1,25-dihydroxyvitamin D3 from human VDR by HAP assayMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Displacement of [3H]1,25-dihydroxyvitamin D3 from human VDR by HAP assayMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Displacement of [3H]1,25-dihydroxyvitamin D3 from human VDR by HAP assayMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Displacement of [3H]1,25-dihydroxyvitamin D3 from human VDR by HAP assayMore data for this Ligand-Target Pair