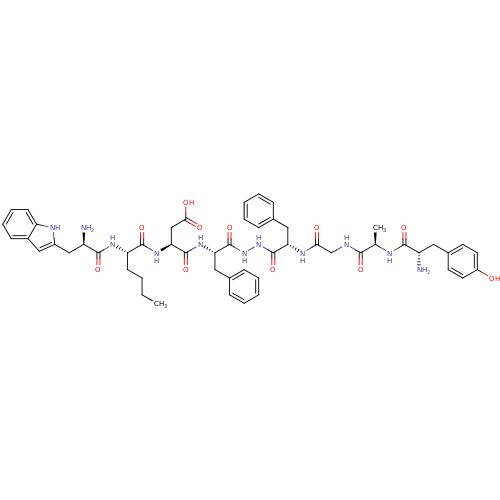
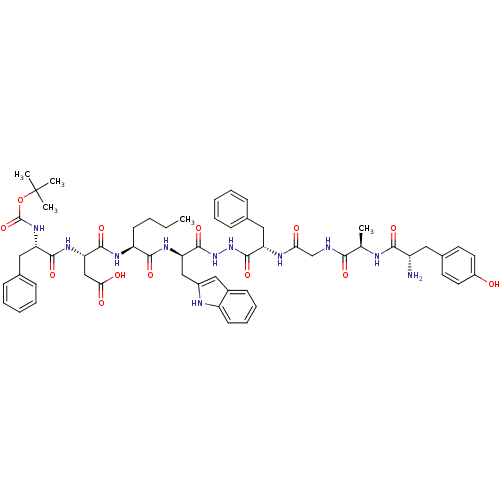
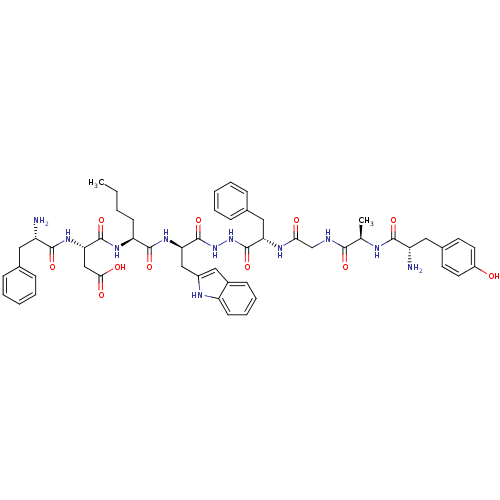
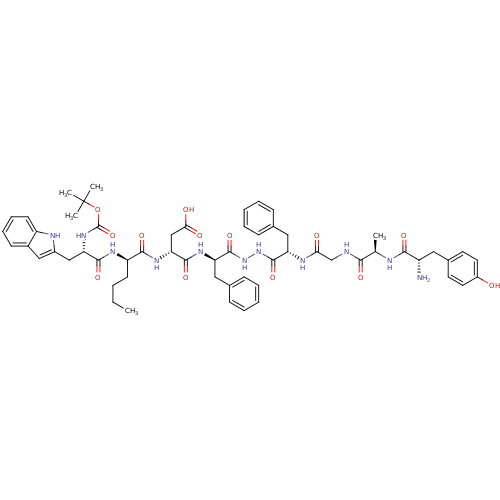
Affinity DataKi: 0.5nMAssay Description:Displacement of [3H]DAMGO from rat mu opioid receptor expressed in HN9.10 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Activity at rat mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.630nMAssay Description:Displacement of [3H]DPDPE from human delta opioid receptor expressed in HN9.10 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Displacement of [3H]DPDPE from human delta opioid receptor expressed in HN9.10 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Activity at rat mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.40nMAssay Description:Activity at rat mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.40nMAssay Description:Displacement of [3H]DAMGO from rat mu opioid receptor expressed in HN9.10 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Displacement of [3H]DAMGO from rat mu opioid receptor expressed in HN9.10 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Activity at rat mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 31nMAssay Description:Activity at rat mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 34nMAssay Description:Displacement of [3H]DAMGO from rat mu opioid receptor expressed in HN9.10 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 34nMAssay Description:Activity at rat mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 45nMAssay Description:Displacement of [3H]DPDPE from human delta opioid receptor expressed in HN9.10 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 52nMAssay Description:Displacement of [3H]DAMGO from rat mu opioid receptor expressed in HN9.10 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 52nMAssay Description:Activity at rat mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 56nMAssay Description:Activity at rat mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
University Of Arizona
Curated by ChEMBL
University Of Arizona
Curated by ChEMBL
Affinity DataKi: 71nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
University Of Arizona
Curated by ChEMBL
University Of Arizona
Curated by ChEMBL
Affinity DataKi: 71nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
University Of Arizona
Curated by ChEMBL
University Of Arizona
Curated by ChEMBL
Affinity DataKi: 1.10E+3nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
University Of Arizona
Curated by ChEMBL
University Of Arizona
Curated by ChEMBL
Affinity DataKi: 1.10E+3nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.90E+3nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 7.00E+3nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
University Of Arizona
Curated by ChEMBL
University Of Arizona
Curated by ChEMBL
Affinity DataKi: 7.70E+3nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
University Of Arizona
Curated by ChEMBL
University Of Arizona
Curated by ChEMBL
Affinity DataKi: 7.70E+3nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 8.30E+3nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
University Of Arizona
Curated by ChEMBL
University Of Arizona
Curated by ChEMBL
Affinity DataKi: 9.30E+3nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
University Of Arizona
Curated by ChEMBL
University Of Arizona
Curated by ChEMBL
Affinity DataKi: 9.30E+3nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
University Of Arizona
Curated by ChEMBL
University Of Arizona
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
University Of Arizona
Curated by ChEMBL
University Of Arizona
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
University Of Arizona
Curated by ChEMBL
University Of Arizona
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
University Of Arizona
Curated by ChEMBL
University Of Arizona
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
University Of Arizona
Curated by ChEMBL
University Of Arizona
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Activity at human delta opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Activity at rat mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Displacement of [3H]DPDPE from human delta opioid receptor expressed in HN9.10 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Activity at rat mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:Activity at delta opioid receptor in ICR mouse by MVD assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Displacement of [3H]DPDPE from human delta opioid receptor expressed in HN9.10 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 4.45nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
University Of Arizona
Curated by ChEMBL
University Of Arizona
Curated by ChEMBL
Affinity DataIC50: 4.60nMAssay Description:Activity at human CCK2 receptor expressed in HEK293 cells assessed as level of [3H]inositol produced relative to controlMore data for this Ligand-Target Pair
Affinity DataIC50: 4.99nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.05nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.10nMAssay Description:Displacement of [3H]DPDPE from human delta opioid receptor expressed in HN9.10 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.15nMAssay Description:Displacement of [125I]CCK8-SO3 from human CCK1 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair