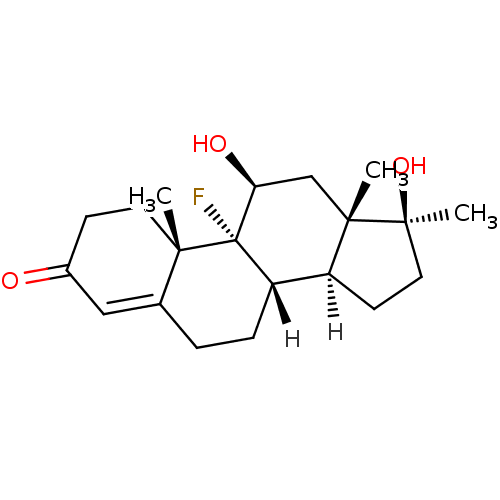
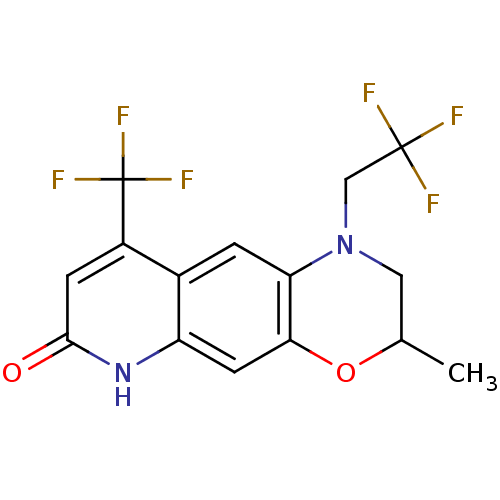
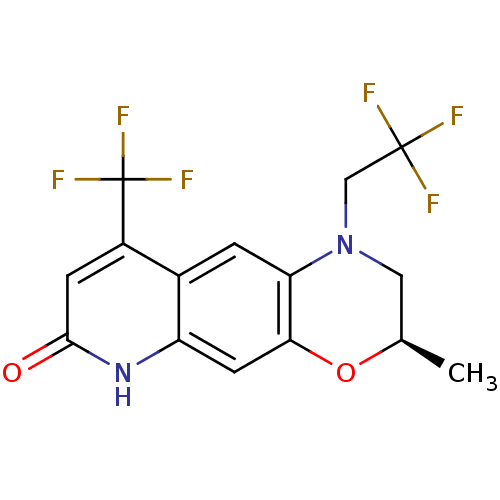
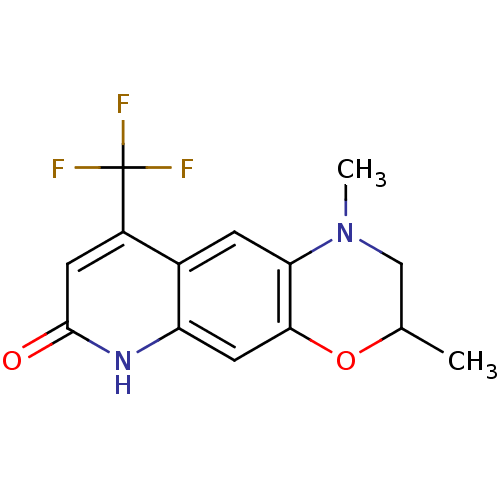
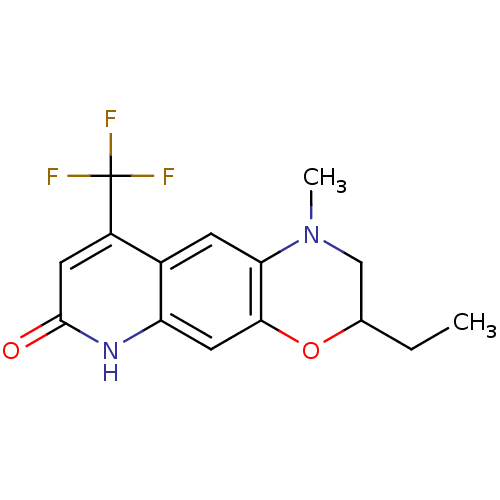
Affinity DataKi: 0.300nM ΔG°: -56.5kJ/mole EC50: 5.70nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
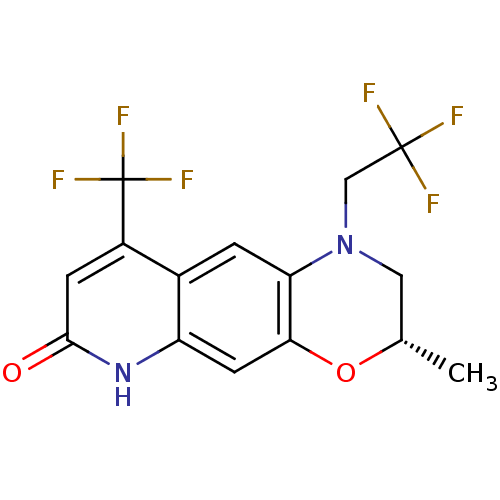
Affinity DataKi: 1.20nM ΔG°: -53.0kJ/mole EC50: 6.40nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
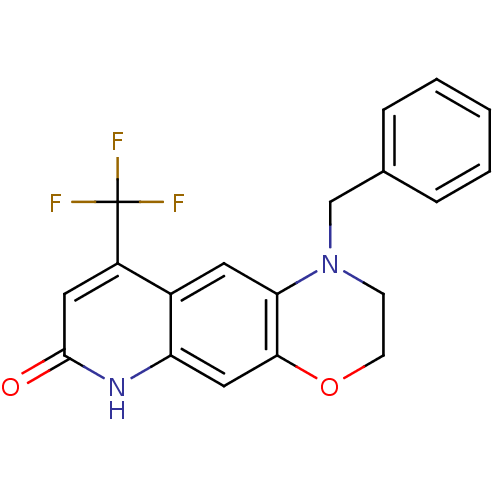
Affinity DataKi: 1.60nM ΔG°: -52.2kJ/mole EC50: 1.40nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
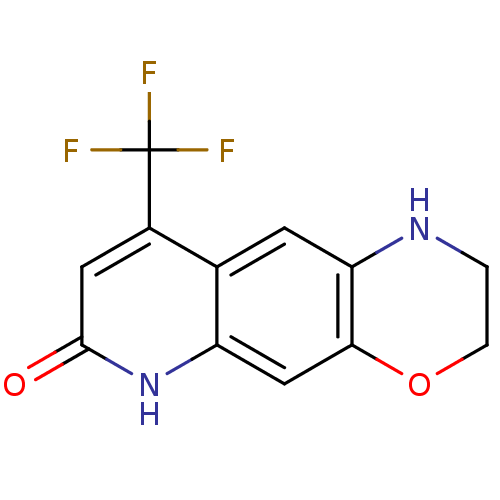
Affinity DataKi: 2.80nM EC50: 2.70nMAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 4nM ΔG°: -49.9kJ/mole EC50: 3.5nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 4nM EC50: 1.90nMAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 4.20nM EC50: 10nMAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 5.60nM EC50: 3.90nMAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 5.70nM EC50: 3.80nMAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 5.70nM ΔG°: -48.9kJ/mole EC50: 0.300nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 7.10nM EC50: 1.10nMAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 7.80nM EC50: 2.70nMAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 9.40nM EC50: 6nMAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 9.60nM ΔG°: -47.6kJ/mole EC50: 2.40nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 11nM ΔG°: -47.3kJ/mole EC50: 4.10nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 15nM ΔG°: -46.5kJ/mole EC50: 18nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 15nM ΔG°: -46.5kJ/mole EC50: 8.90nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 16nM EC50: 14nMAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 17nM ΔG°: -46.1kJ/mole EC50: 3.20nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 19nM EC50: 30nMAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 23nM EC50: 2.30nMAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 25nM ΔG°: -45.1kJ/mole EC50: 7.80nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 27nM EC50: 26nMAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 30nM ΔG°: -44.7kJ/mole EC50: 15nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 33nM ΔG°: -44.4kJ/mole EC50: 47nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 34nM ΔG°: -44.3kJ/mole EC50: 31nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 48nM ΔG°: -43.5kJ/mole EC50: 7.80nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 51nM EC50: 5.90nMAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 58nM ΔG°: -43.0kJ/mole IC50: 67nM EC50: 52nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 75nM ΔG°: -42.3kJ/mole EC50: 44nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 109nM EC50: 27nMAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 113nM EC50: 133nMAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 206nM ΔG°: -39.7kJ/mole IC50: 224nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nM ΔG°: >-35.6kJ/mole IC50: 296nMpH: 7.4 T: 2°CAssay Description:The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece...More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nM ΔG°: -30.2kJ/molepH: 7.4 T: 2°CAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 2.80E+3nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 2.80E+3nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 5.20E+3nM ΔG°: -28.0kJ/molepH: 7.4 T: 2°CAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 5.30E+3nM ΔG°: -28.0kJ/molepH: 7.4 T: 2°CAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 5.40E+3nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 6.50E+3nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 7.00E+3nM ΔG°: -27.4kJ/molepH: 7.4 T: 2°CAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 8.10E+3nM ΔG°: -27.0kJ/molepH: 7.4 T: 2°CAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 8.50E+3nMAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 8.80E+3nM ΔG°: -26.8kJ/molepH: 7.4 T: 2°CAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair
Affinity DataKi: 9.20E+3nM ΔG°: -26.7kJ/molepH: 7.4 T: 2°CAssay Description:The procedure for the cross reactivity receptor binding assays was using baculovirus-expressed receptors. After correcting for nonspecific binding, I...More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)