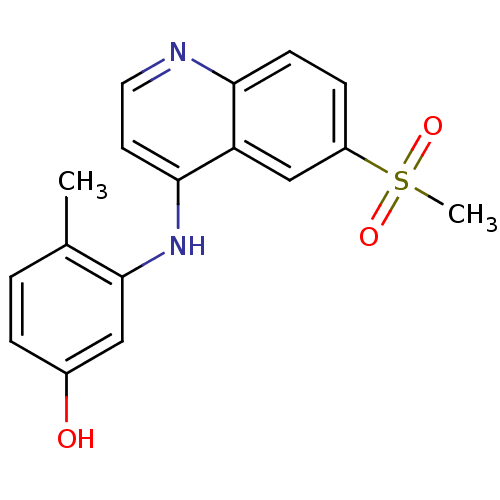
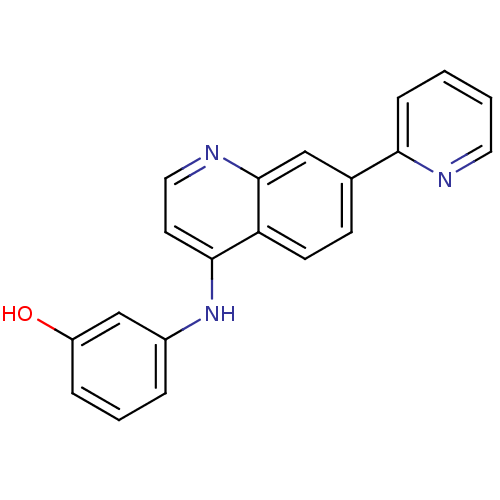
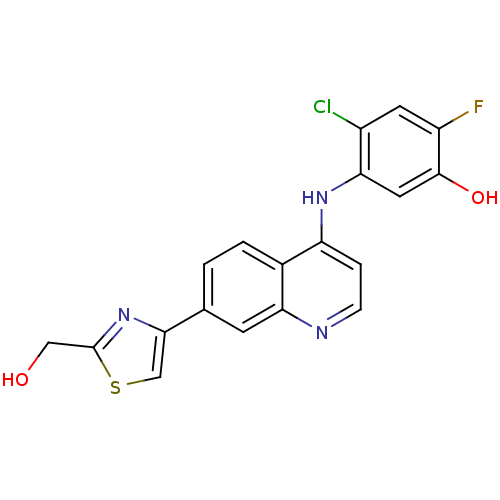
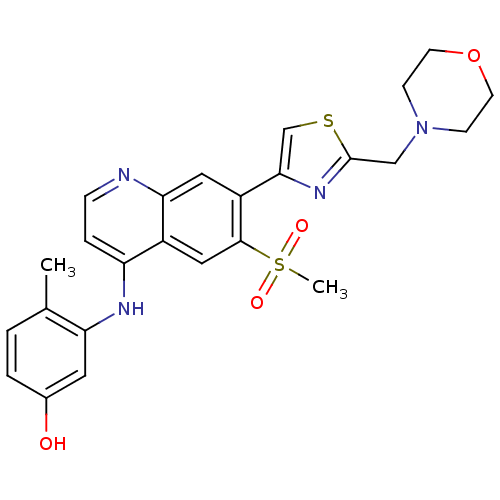
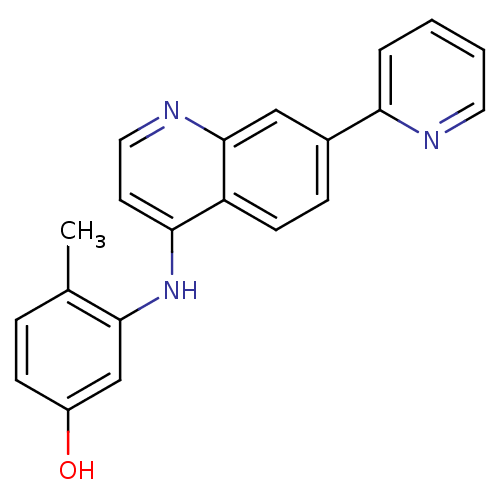
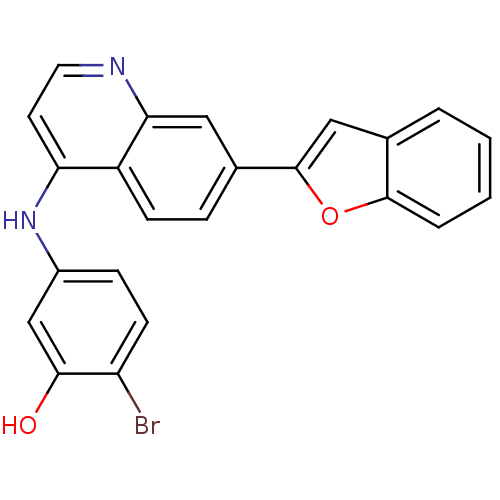
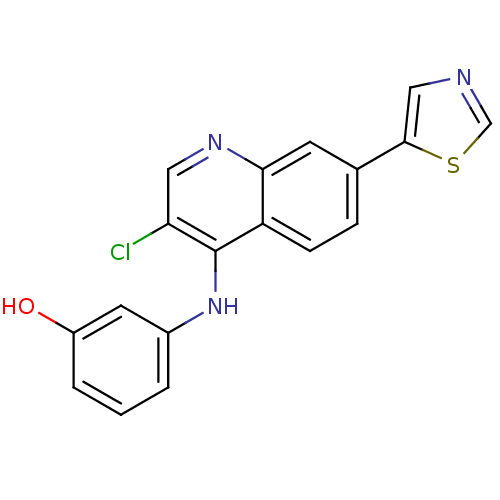
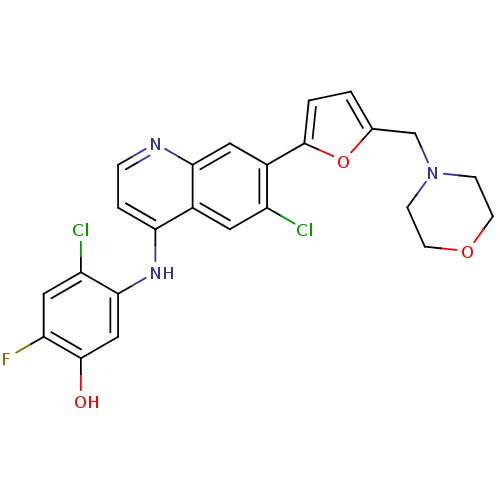
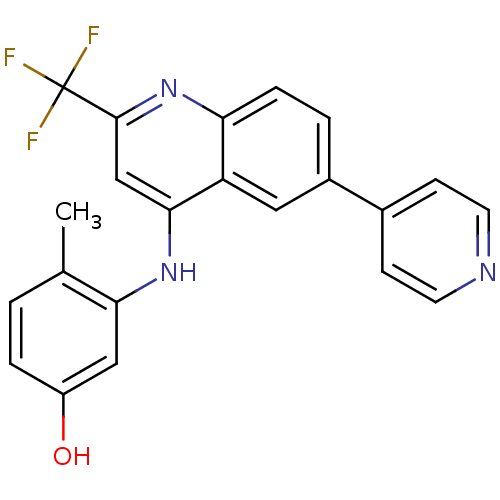
Affinity DataIC50: 3nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 25nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 25nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 34nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 75nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 75nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 80nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 85nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 90nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 95nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMpH: 7.4 T: 2°CAssay Description:The biochemical activity of compound was determined by incubation with Ret kinase and the substrate in the presence of ATP/ [gamma-33P] ATP. After in...More data for this Ligand-Target Pair