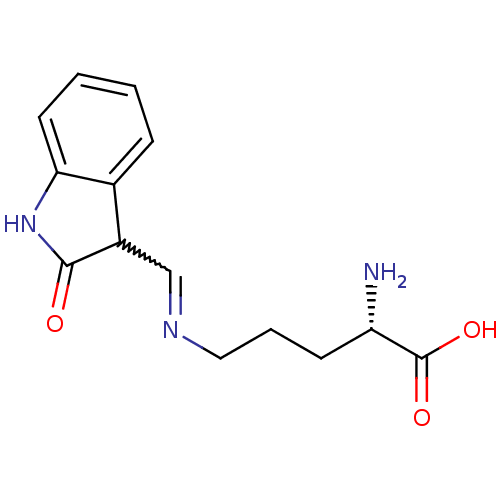
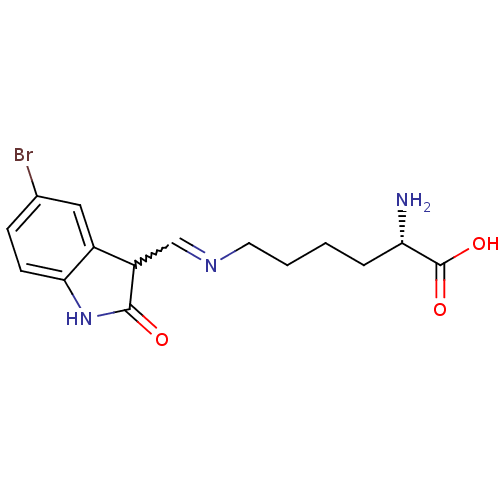
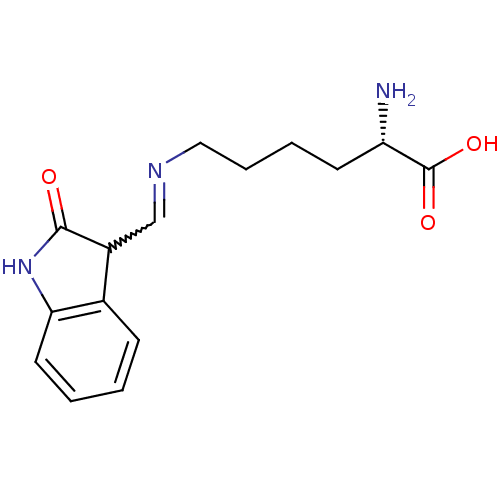
Affinity DataIC50: 700nMAssay Description:Inhibition of CDK2/Cyclin A (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 6.60E+3nMAssay Description:Inhibition of CDK2/Cyclin A (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of cMet (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of cMet (unknown origin)More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Université
Curated by ChEMBL
Université
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Ret (unknown origin)More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Université
Curated by ChEMBL
Université
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Ret (unknown origin)More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Université
Curated by ChEMBL
Université
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Ret (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Src (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Src (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Src (unknown origin)More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Université
Curated by ChEMBL
Université
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of KDR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of IGF1R (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of cMet (unknown origin)More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Université
Curated by ChEMBL
Université
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Ret (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Src (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of c-Abl (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of c-Abl (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of c-Abl (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of c-Abl (unknown origin)More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Université
Curated by ChEMBL
Université
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of KDR (unknown origin)More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Université
Curated by ChEMBL
Université
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of KDR (unknown origin)More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Université
Curated by ChEMBL
Université
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of KDR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of IGF1R (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of IGF1R (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of IGF1R (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of cMet (unknown origin)More data for this Ligand-Target Pair

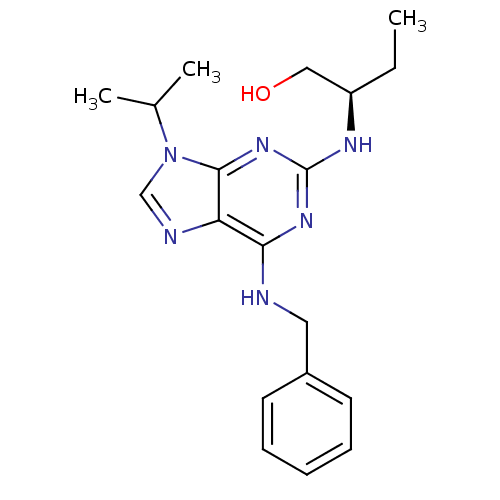
 3D Structure (crystal)
3D Structure (crystal)