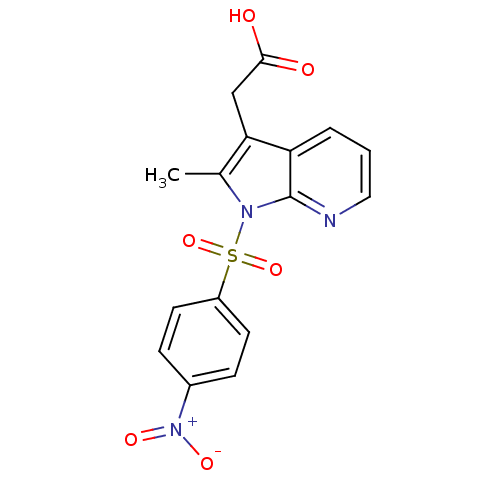
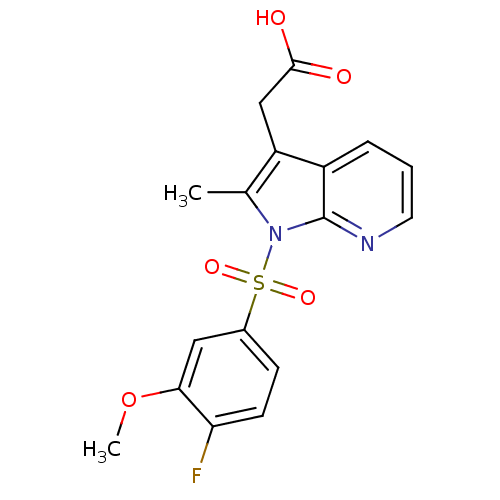
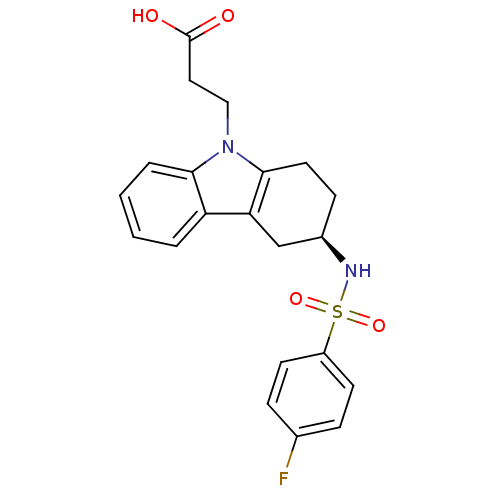
TargetProstaglandin D2 receptor 2(Mus musculus (mouse))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: 28nMAssay Description:Displacement of [3H]PGD2 from mouse CRTh2 receptor expressed in K562 cellsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: 31nMAssay Description:Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: 35nMAssay Description:Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: 36nMAssay Description:Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: 43nMAssay Description:Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: 48nMAssay Description:Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: 52nMAssay Description:Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: 56nMAssay Description:Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: 59nMAssay Description:Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: 73nMAssay Description:Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: 103nMAssay Description:Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: 158nMAssay Description:Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: 282nMAssay Description:Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: 377nMAssay Description:Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: 619nMAssay Description:Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: 1.17E+3nMAssay Description:Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: 2.62E+3nMAssay Description:Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: 3.22E+3nMAssay Description:Displacement of [3H]PGD2 from human CRTh2 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity to human prostaglandin D2 receptorMore data for this Ligand-Target Pair
TargetThromboxane A2 receptor(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity to human thromboxane A2 receptorMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Antagonist activity against CRTh2 receptor in human eosinophils assessed as cell shape changeMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Antagonist activity against CRTh2 receptor in human eosinophils assessed as cell shape changeMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Antagonist activity against CRTh2 receptor in human eosinophils assessed as cell shape changeMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Antagonist activity against CRTh2 receptor in human eosinophils assessed as cell shape changeMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Antagonist activity against CRTh2 receptor in human whole blood assessed as eosinophil shape changeMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Antagonist activity against CRTh2 receptor in human eosinophils assessed as cell shape changeMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Antagonist activity against CRTh2 receptor in human eosinophils assessed as cell shape changeMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 31nMAssay Description:Antagonist activity against human CRTh2 receptor expressed in CHO cells assessed as effect on cAMP accumulationMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Antagonist activity against CRTh2 receptor in human whole blood assessed as eosinophil shape changeMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 41nMAssay Description:Antagonist activity against human CRTh2 receptor expressed in CHO cells assessed as effect on cAMP accumulationMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Antagonist activity against CRTh2 receptor in human eosinophils assessed as cell shape changeMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 56nMAssay Description:Antagonist activity against CRTh2 receptor in human whole blood assessed as eosinophil shape changeMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Antagonist activity against human CRTh2 receptor expressed in CHO cells assessed as effect on cAMP accumulationMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 91nMAssay Description:Antagonist activity against human CRTh2 receptor expressed in CHO cells assessed as effect on cAMP accumulationMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 101nMAssay Description:Antagonist activity against human CRTh2 receptor expressed in CHO cells assessed as effect on cAMP accumulationMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 108nMAssay Description:Antagonist activity against CRTh2 receptor in human eosinophils assessed as cell shape changeMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 112nMAssay Description:Antagonist activity against CRTh2 receptor in human eosinophils assessed as cell shape changeMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 148nMAssay Description:Antagonist activity against human CRTh2 receptor expressed in CHO cells assessed as effect on cAMP accumulationMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 156nMAssay Description:Antagonist activity against CRTh2 receptor in human whole blood assessed as eosinophil shape changeMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Mus musculus (mouse))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 167nMAssay Description:Antagonist activity against mouse CRTh2 receptor expressed in K562 cells by [35S]GTPgamma binding assayMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 195nMAssay Description:Antagonist activity against CRTh2 receptor in human whole blood assessed as eosinophil shape changeMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Antagonist activity against human CRTh2 receptor expressed in CHO cells assessed as effect on cAMP accumulationMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 248nMAssay Description:Antagonist activity against CRTh2 receptor in human eosinophils assessed as cell shape changeMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 311nMAssay Description:Antagonist activity against human CRTh2 receptor expressed in CHO cells assessed as effect on cAMP accumulationMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 354nMAssay Description:Antagonist activity against human CRTh2 receptor expressed in CHO cells assessed as effect on cAMP accumulationMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor 2(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 629nMAssay Description:Antagonist activity against CRTh2 receptor in human whole blood assessed as eosinophil shape changeMore data for this Ligand-Target Pair
TargetCholecystokinin receptor type A(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 8.10E+3nMAssay Description:Inhibition of CCKalpha receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP2 subtype(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Antagonist activity against prostaglandin E2 receptorMore data for this Ligand-Target Pair
TargetProstaglandin D2 receptor(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Binding affinity to human prostaglandin D2 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP3 subtype(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Antagonist activity against prostaglandin E3 receptorMore data for this Ligand-Target Pair