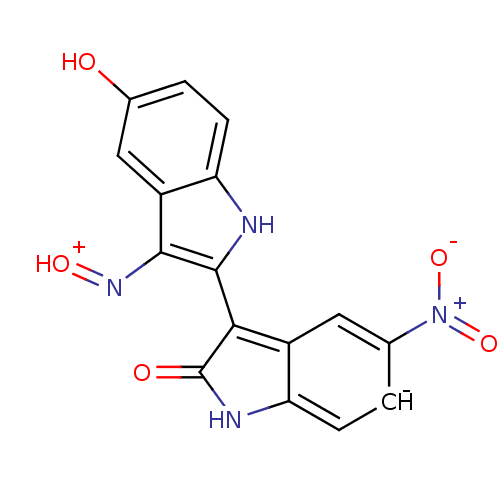
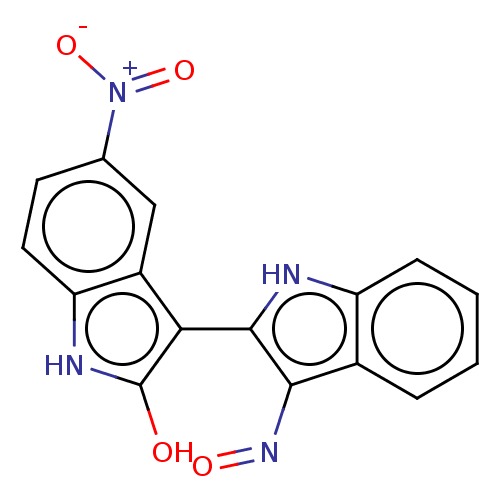
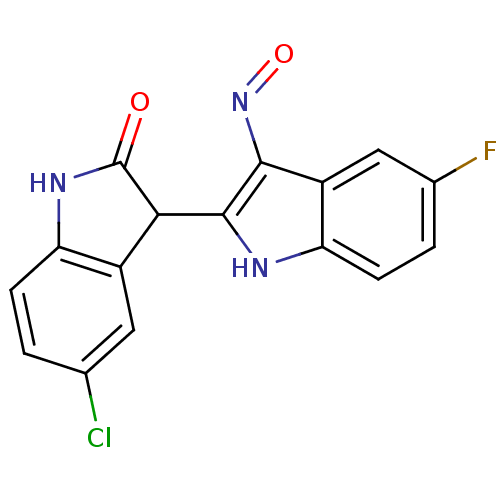
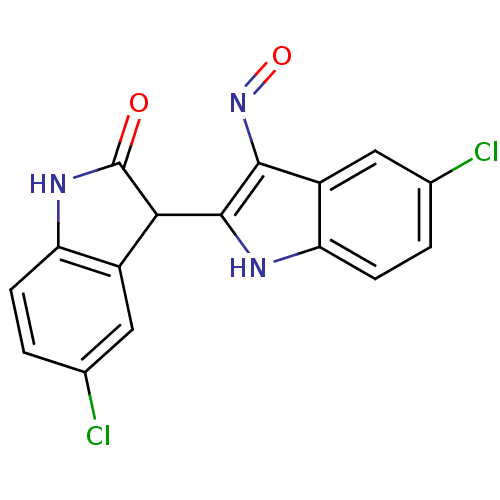
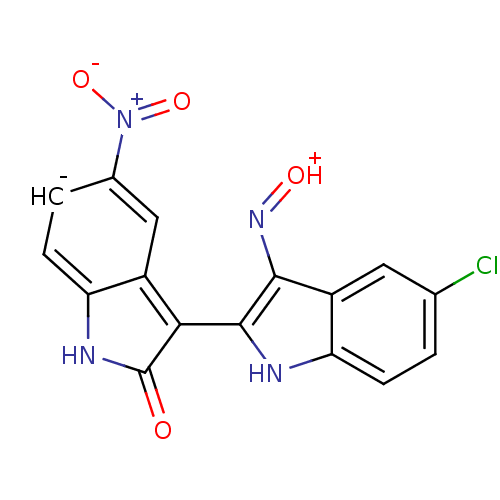
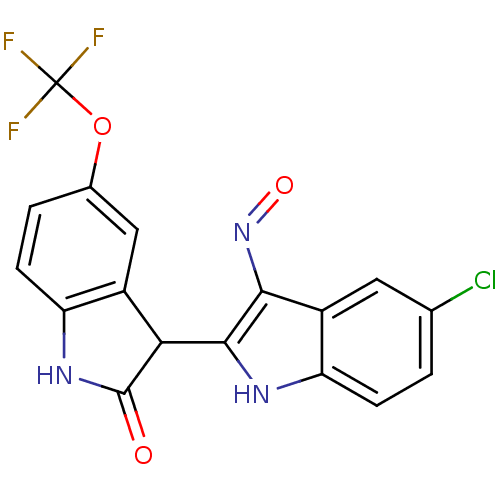
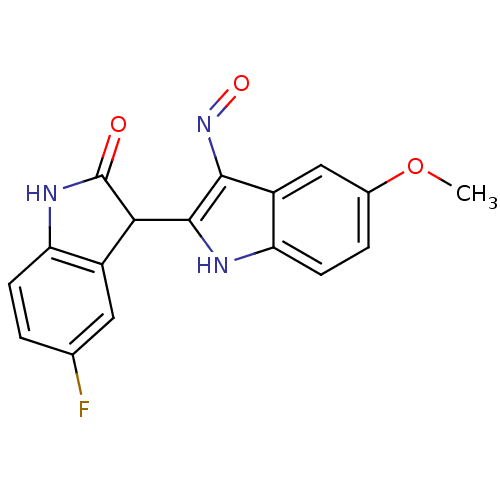
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Inhibition of CDK2/cyclin EMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of CDK2/cyclin EMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Inhibition of CDK2/cyclin EMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Inhibition of CDK2/cyclin EMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 5.30nMAssay Description:Inhibition of CDK2/cyclin EMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 7.40nMAssay Description:Inhibition of CDK2/cyclin EMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of CDK2/cyclin EMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 8.30nMAssay Description:Inhibition of CDK2/cyclin EMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 8.70nMAssay Description:Inhibition of CDK2/cyclin EMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of CDK2/cyclin EMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of CDK2/cyclin EMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of CDK1/cyclin B after 40 mins by liquid scintillation countingMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 24nMAssay Description:Inhibition of CDK2/cyclin EMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 60nMAssay Description:Inhibition of CDK2/cyclin EMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 76nMAssay Description:Inhibition of CDK2/cyclin EMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 195nMAssay Description:Inhibition of CDK1/cyclin B after 40 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of recombinant auroraA preincubated for 15 mins by HTRF assayMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 2.95E+3nMAssay Description:Inhibition of CDK2/cyclin EMore data for this Ligand-Target Pair
TargetHigh affinity nerve growth factor receptor(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of recombinant TrKa preincubated for 15 mins by HTRF assayMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 4.12E+3nMAssay Description:Inhibition of CDK2/cyclin EMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 2/G1/S-specific cyclin-E1(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 8.62E+3nMAssay Description:Inhibition of CDK2/cyclin EMore data for this Ligand-Target Pair
TargetInhibitor of nuclear factor kappa-B kinase subunit beta(Homo sapiens (Human))
Institute Of Science And Technology
Curated by ChEMBL
Institute Of Science And Technology
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of recombinant IKK-beta preincubated for 15 mins by HTRF assayMore data for this Ligand-Target Pair