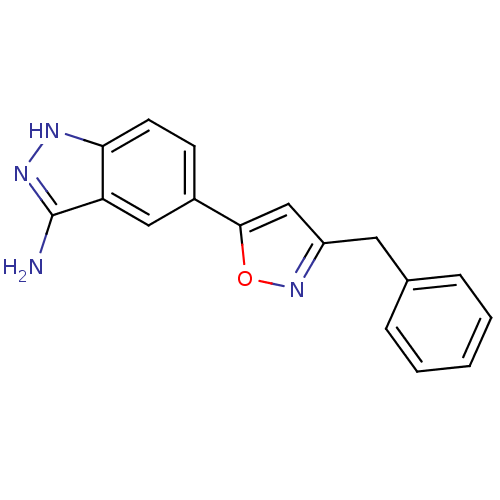
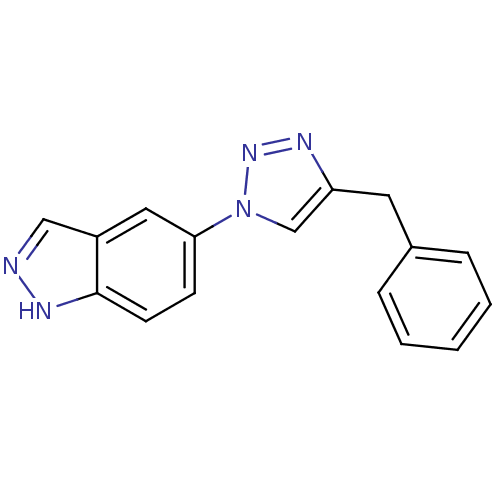
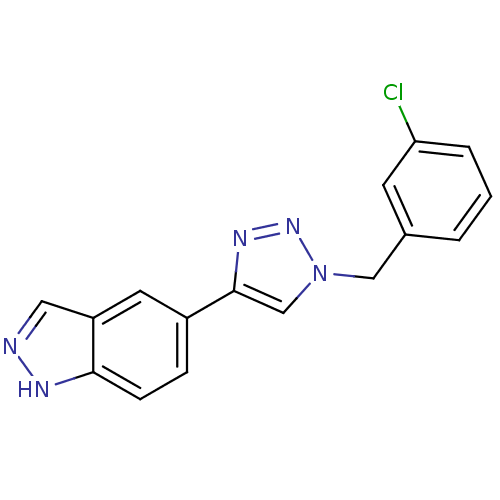
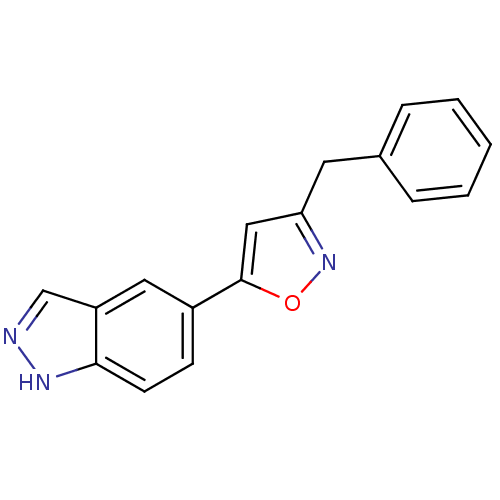
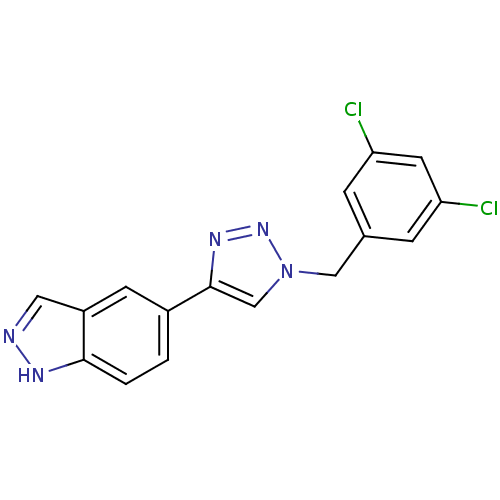
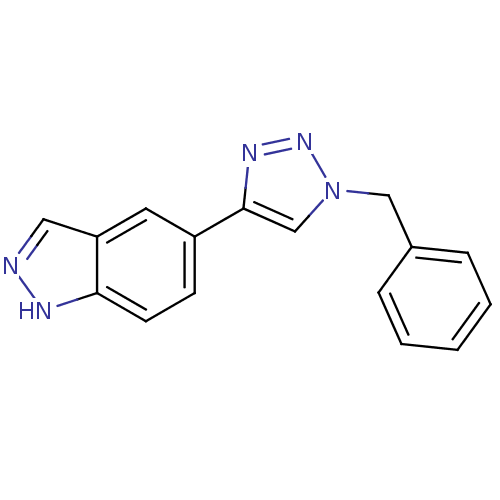
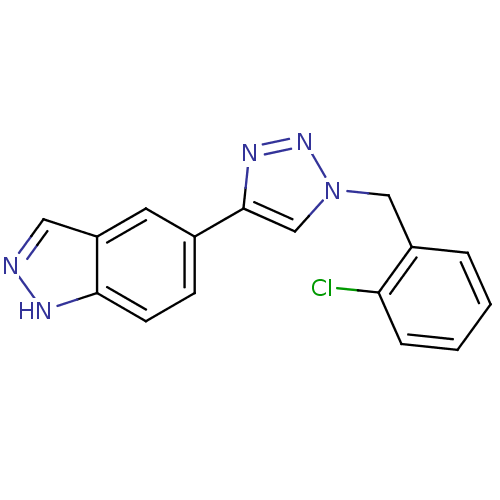
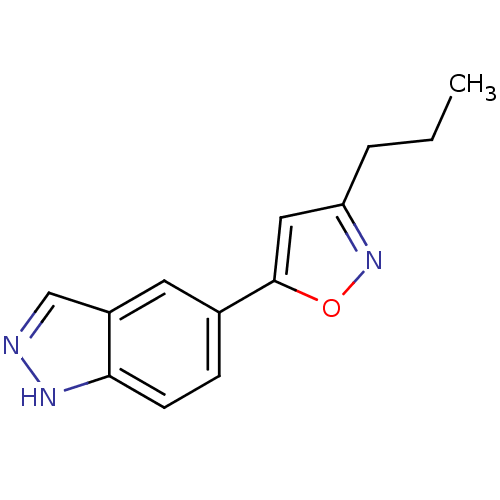
Affinity DataKi: 15nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Inhibition of Aurora kinase 2 after 1 hr using biotinylated kemptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Inhibition of GSK3-beta after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Inhibition of Aurora kinase 2 after 1 hr using biotinylated kemptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Inhibition of JAK2 after 1 hr using biotinylated PDKtide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 19nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Inhibition of GSK3-beta after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Inhibition of GSK3-beta after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Inhibition of Aurora kinase 2 after 1 hr using biotinylated kemptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 28nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 28nMAssay Description:Inhibition of GSK3-beta after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 39nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 39nMAssay Description:Inhibition of GSK3-beta after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 41nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 42nMAssay Description:Inhibition of Aurora kinase 2 after 1 hr using biotinylated kemptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 44nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 45nMAssay Description:Inhibition of GSK3-beta after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 48nMAssay Description:Inhibition of GSK3-beta after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 62nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 64nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 66nMAssay Description:Inhibition of Aurora kinase 2 after 1 hr using biotinylated kemptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 68nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 70nMAssay Description:Inhibition of GSK3-beta after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 70nMAssay Description:Inhibition of GSK3-beta after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 73nMAssay Description:Inhibition of Aurora kinase 2 after 1 hr using biotinylated kemptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 91nMAssay Description:Inhibition of GSK3-beta after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 91nMAssay Description:Inhibition of JAK2 after 1 hr using biotinylated PDKtide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 97nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 97nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 108nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 113nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Inhibition of Aurora kinase 2 after 1 hr using biotinylated kemptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 180nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 186nMAssay Description:Inhibition of Aurora kinase 2 after 1 hr using biotinylated kemptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 197nMAssay Description:Inhibition of GSK3-beta after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 209nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 230nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 240nMAssay Description:Inhibition of Aurora kinase 2 after 1 hr using biotinylated kemptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 252nMAssay Description:Inhibition of Aurora kinase 2 after 1 hr using biotinylated kemptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 265nMAssay Description:Inhibition of GSK3-beta after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 281nMAssay Description:Inhibition of JAK2 after 1 hr using biotinylated PDKtide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 284nMAssay Description:Inhibition of JAK2 after 1 hr using biotinylated PDKtide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 285nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 285nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 315nMAssay Description:Inhibition of Rock2 after 1 hr using biotinylated longS peptide as substrateMore data for this Ligand-Target Pair
Affinity DataKi: 323nMAssay Description:Inhibition of Aurora kinase 2 after 1 hr using biotinylated kemptide as substrateMore data for this Ligand-Target Pair