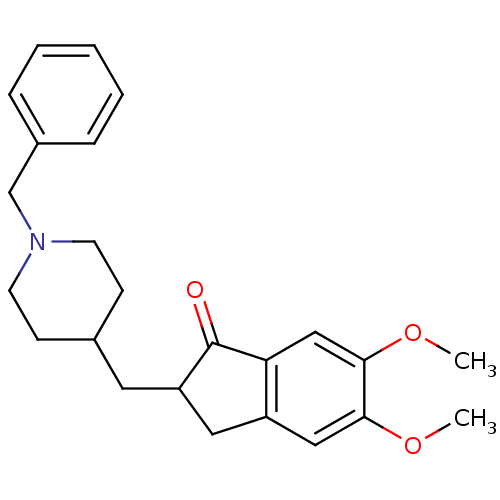
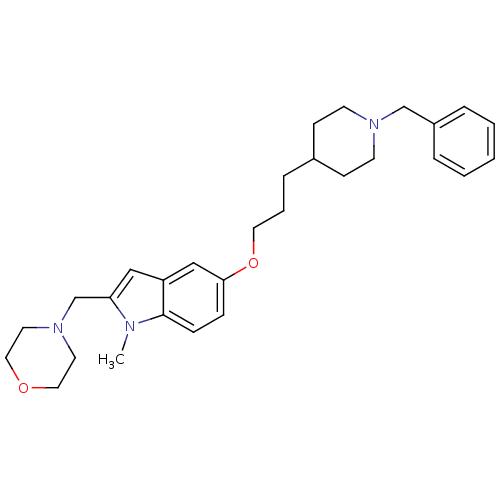
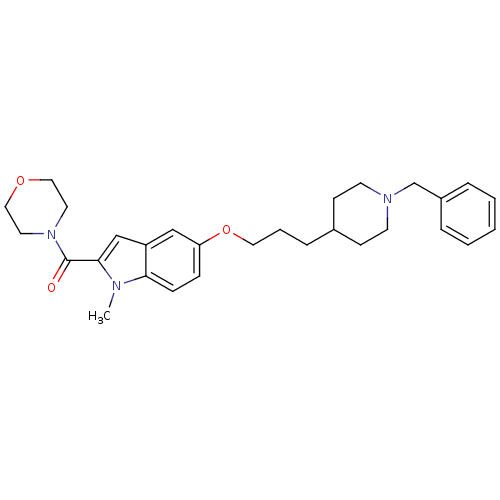
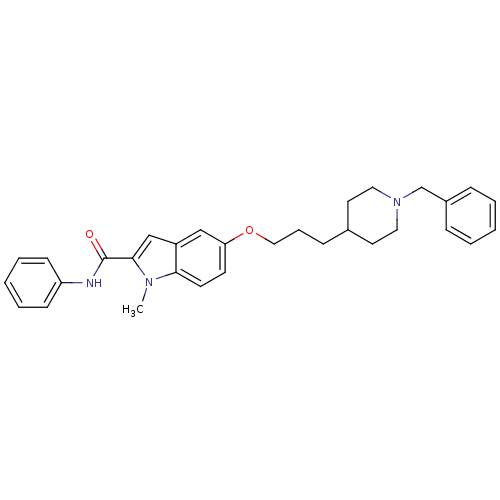
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 84nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 84nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 130nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 140nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 190nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 190nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 240nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 270nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 270nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 290nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 290nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 350nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 400nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 460nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 460nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 460nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 790nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 830nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 830nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 840nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 849nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 850nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 1.52E+4nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 1.52E+4nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 2.29E+4nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: 2.29E+4nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after 5 ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Laboratorio De Quimica Medica (Iqog, Csic)
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair