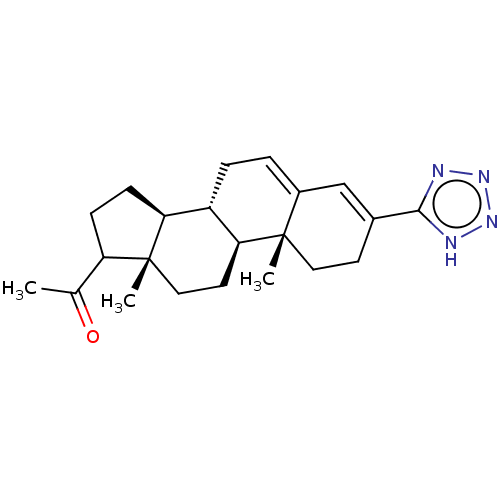
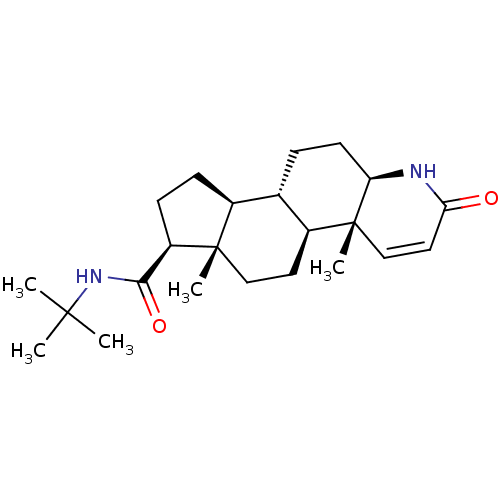
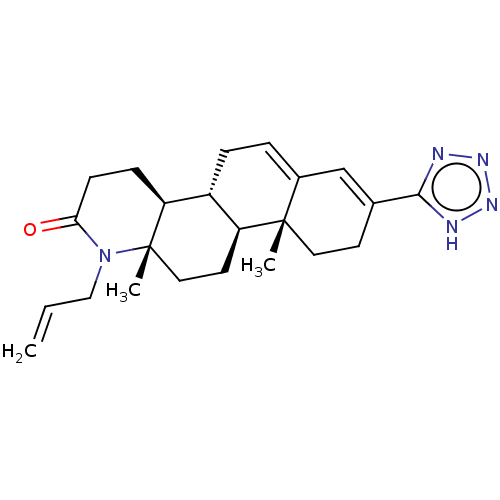
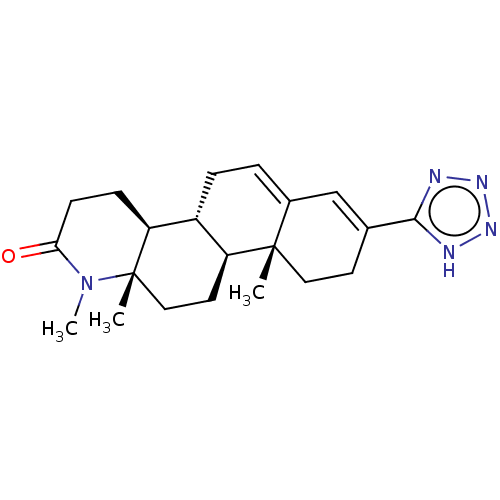
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Institute Of Pharmaceutical Sciences
Curated by ChEMBL
University Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of human 5alpha-2 reductase expressed in HEK293 cells assessed as suppression of conversion of [3]androstenedione incubated for 30 mins by...More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Institute Of Pharmaceutical Sciences
Curated by ChEMBL
University Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of human 5alpha-2 reductase expressed in HEK293 cells assessed as suppression of conversion of [3]androstenedione incubated for 30 mins by...More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Institute Of Pharmaceutical Sciences
Curated by ChEMBL
University Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 84nMAssay Description:Inhibition of human 5alpha-2 reductase expressed in HEK293 cells assessed as suppression of conversion of [3]androstenedione incubated for 30 mins by...More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Institute Of Pharmaceutical Sciences
Curated by ChEMBL
University Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 157nMAssay Description:Inhibition of human 5alpha-2 reductase expressed in HEK293 cells assessed as suppression of conversion of [3]androstenedione incubated for 30 mins by...More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Institute Of Pharmaceutical Sciences
Curated by ChEMBL
University Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 220nMAssay Description:Inhibition of human 5alpha-2 reductase expressed in HEK293 cells assessed as suppression of conversion of [3]androstenedione incubated for 30 mins by...More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 2(Homo sapiens (Human))
University Institute Of Pharmaceutical Sciences
Curated by ChEMBL
University Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 274nMAssay Description:Inhibition of human 5alpha-2 reductase expressed in HEK293 cells assessed as suppression of conversion of [3]androstenedione incubated for 30 mins by...More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Institute Of Pharmaceutical Sciences
Curated by ChEMBL
University Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 453nMAssay Description:Inhibition of human 5alpha-1 reductase expressed in HEK293 cells assessed as suppression of conversion of [3]androstenedione incubated for 30 mins by...More data for this Ligand-Target Pair
Target3-oxo-5-alpha-steroid 4-dehydrogenase 1(Homo sapiens (Human))
University Institute Of Pharmaceutical Sciences
Curated by ChEMBL
University Institute Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataIC50: 547nMAssay Description:Inhibition of human 5alpha-1 reductase expressed in HEK293 cells assessed as suppression of conversion of [3]androstenedione incubated for 30 mins by...More data for this Ligand-Target Pair